An emmetropic 42 year old immunocompetent woman with 6/6 vision developed left eye pain while wearing cosmetic soft contact lenses. She presented on 28 July 2000 to her ophthalmologist, who noted deep stromal infiltration accompanying a 2 × 3 mm pericentral corneal ulcer. Cultures yielded Staphylococcus aureus, Streptococcus viridans, and Fusarium solani. Initial therapy with tobramycin was followed by high dose topical hydroquinolones, whereafter the infection, continuing unabated, was construed to be fungal keratitis. High doses of amphotericin B both topical and intravenously, natamycin, and ketoconazole were administered, along with topical fortified cephazolin, neosporin, and atropine. Despite these, the lesion spread to involve much of the corneal periphery (Fig 1), and repeat corneal cultures confirmed the presence of amphotericin B resistant Fusarium spp (MIC 24:48 hours in μg/ml: amphotericin B 2:2; natamycin 32:32; posaconazole 1:8).
Figure 1.
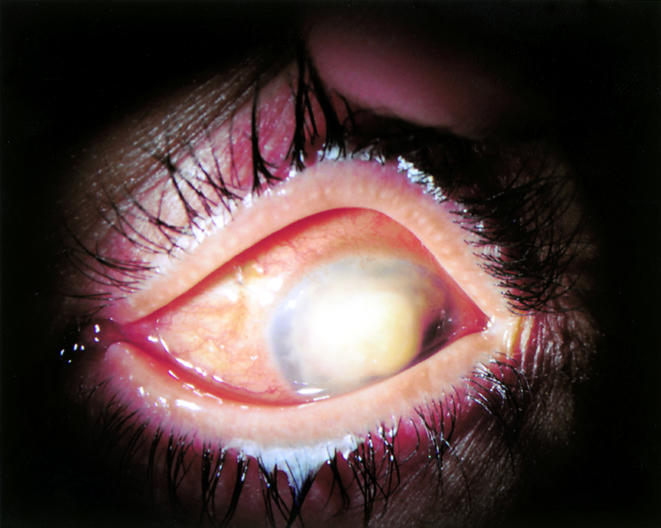
Left eye of 42 year old woman with invasive Fusarium solani keratitis/endophthalmitis after 8 weeks of maximal tolerated therapy with natamycin and amphotericin B.
On 1 September 2000, the anterior chamber was filled with fibrin with a central corneal descemetocele and near total corneal infiltration, affording only a hazy view of the peripheral iris. The fibrin clot was integrated with the central iris, and the lens was not visible. Ultrasonography suggested anterior segment involvement only. Posaconazole (SCH-56592),1–6 an investigational broad spectrum triazole, was obtained from Schering-Plough Research Corporation and administered at 200 mg four times daily orally, with hourly topical ocular application of the same suspension (10 mg/0.1 ml). A compassionate use investigative new drug (IND) approval for topical ocular application of posaconazole was requested from, and promptly issued by, the Federal Drug Administration, and informed consent was obtained through an institutional review board approved protocol. Treatment commenced 5 September 2000, when aqueous tap reconfirmed presence of Fusarium, which was susceptible in vitro to posaconazole.
Within the first week of 2 hourly topical application of posaconazole oral suspension (100 mg/ml), along with 800 mg orally of the same daily, there was significant clearing of the corneal periphery. The fibrin clot melted concentrically, revealing its attachment to the descemetocele anteriorly and central iris posteriorly.
With further clearing of the infectious inflammatory clot, the anterior descemetocele became effectively unplugged. The globe decompressed through the open corneal ulcer providing the impetus for urgent penetrating keratoplasty on 20 September 2000. Histology revealed innumerable septate branching fungi within the corneal and iris stroma.
One week later, further remarkable improvement was noted. Despite the ominous surgical histology, >90% of the fibrin clot had cleared. Diagnostic vitrectomy yielded posaconazole at a concentration of 0.25 μg/ml in the vitreous (and 1.2 μg/ml in the plasma) on 26 September 2000. Vision improved markedly, with good projection to confrontational light throughout the periphery. The clarity of the transplant and anterior chamber now revealed a dense white cortical cataract, with residual fibrin clot inferior to the graft-host interface. Topical corticosteroid therapy was cautiously introduced. By 30 October 2000 the patient's condition was further improved, and elective combined phacoemulsification with planned aphakia was carried out on 11 January 2001. Branching elements of the Fusarium were histologically confirmed to have penetrated the surgically removed anterior lens cortex and capsule, but all subsequent cultures were negative, and aqueous tap confirmed posaconazole to be present at a level of 0.9 μg/ml, with a plasma level of 1.6 μl/ml.
At 16 months, on 4 December 2001, vision remained stable with good colour vision and 360 degree peripheral visual function. The visual axis was clear, with two small clear operculi in the otherwise opaque residual posterior capsule (Fig 2). Visual acuity was 6/30 using aphakic correction, with no afferent pupil defect. Prognosis for eventual lens replacement, posterior capsulotomy, and visual rehabilitation of this eye now appeared very good. This excellent outcome is not anticipated for invasive Fusarium of the eye.7–10
Figure 2.
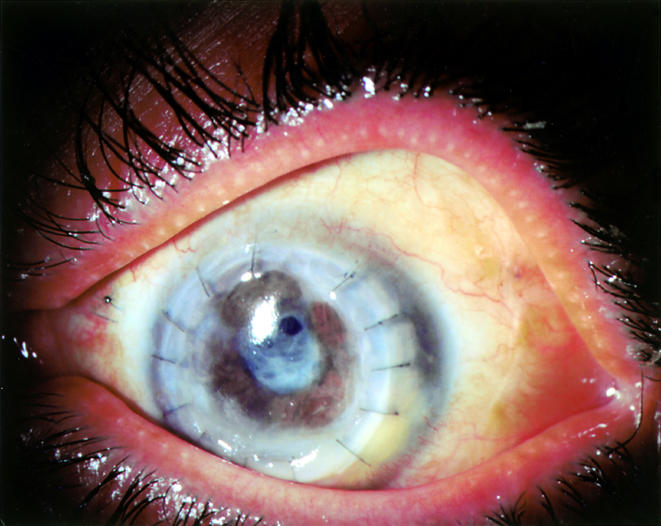
Eye shown in Figure 1 after successful treatment with topical and systemic posaconazole. Status post-penetrating keratoplasty and extracapsular cataract extraction with planned pseudophakia. The removed lens cortex contained fungal hyphae on histopathological assessment.
In summary, a healthy woman with amphotericin and natamycin resistant Fusarium spp keratitis, progressing to invasive endophthalmitis, recovered with good retinal function via an apparently rapid response of the Fusarium to systemic and/or topical posaconazole. The ocular penetration of posaconazole was confirmed on separate occasions by aqueous and vitreous analysis.
Acknowledgments
Investigative new drug evaluation under UTHSCSA IRB P00041 (6/28/98; modified open label treatment protocol for the safety and efficacy of SCH 56592 (posaconazole) in the treatment of refractory mycoses).
The authors gratefully acknowledge the assistance of Gilbert Viprao in coordinating this study, and Schering-Plough for providing the study drug and funding for Mr Viprao.
Supported in part by an unrestricted grant from Research to Prevent Blindness, New York, USA.
The authors have no proprietary interest in any products used in this assessment.
References
- 1.Cacciapuoti A, Loebenberg D, Corcoran E, et al. In vitro and in vivo activities of SCH 56592 (posaconazole), a new triazole antifungal agent, against Aspergillus and Candida. Antimicrob Agents Chemother 2000;44:2017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng W, Liu H, Chen G, et al. Structural characterization of the oxidative degradation products of an antifungal agent SCH 56592 by LC-NMR and LOC-MS. J Pharm Biomed Anal 2001;25:545–57. [DOI] [PubMed] [Google Scholar]
- 3.Walsh TJ, Viviani MA, Arathoon E, et al. New targets and delivery systems for antifungal therapy. Med Mycol 2000;38:335–47. [PubMed] [Google Scholar]
- 4.Graybill JR. Changing strategies for treatment of systemic mycoses. Braz J Inf Dis 2000;4:47–54. [PubMed] [Google Scholar]
- 5.Hossain MA, Ghannoum MA. New investigational antifungal agents for treating invasive fungal infections. Expert Opin Investig Drugs 2000;9:1797–813. [DOI] [PubMed] [Google Scholar]
- 6.Uchida K, Yokota N, Yamaguchi H. In vitro antifungal activity of posaconazole against various pathogenic fungi. Int J Antimicrob Agents 2001;18:167–72. [DOI] [PubMed] [Google Scholar]
- 7.Cheikh-Rouhou F, Makni F, Ayadi A, et al. Ocular parasitoses and mycoses: cases diagnosed in the Central University Hospital of Sfax between 1996 and 1999. Bull Soc Pathol Exot 2001;94:11–13. [PubMed] [Google Scholar]
- 8.Gupta V, Dada T, Vajpayee RB, et al. Polymicrobial keratitis after laser in situ keratomileusis. J Refract Surg 2000;17:147–8. [DOI] [PubMed] [Google Scholar]
- 9.Foroozan R, Eagle RC Jr, Cohen EJ. Fungal keratitis in a soft contact lens wearer. CLAO J 2000;26:166–8. [PubMed] [Google Scholar]
- 10.Guarro J, Pujol I, Mayayo E. In vitro and in vivo experimental activities of antifungal agents against Fusarium solani. Antimicrob Agents Chemother 1999;43:1256–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


