Abstract
Aims: To evaluate in vivo fibroplasia and biological stability of porous polymers intended for use in the Seoul-type keratoprosthesis (S-KPro).
Methods: Four porous polymers (polypropylene, two kinds of polyethylene terephthalate (PE70 and PE50), and polyurethane) were investigated. Discs of polymers were inserted into the corneal stroma of rabbits for a 2 and 5 month period. Corneal oedema and neovascularisation were evaluated. The fibroplasia and collagen deposition were examined under light and transmission electron microscopy. S-KPros, whose skirt was made of four types of polymer, were implanted into the rabbits' eyes. The retention time and complications were evaluated.
Results: Neovascularisation and corneal oedema were found in all of the disc inserted eyes, but the corneal oedema subsided within 2 months in most of the eyes. The mean number of fibroblasts increased significantly in polypropylene and PE50 disc inserted eyes compared with polyurethane disc inserted eyes. Plentiful collagen deposition was also found in both polypropylene and PE50 disc inserted eyes. Mean retention time in the polypropylene SK-Pro implanted eyes was longer than that of the other eyes (20.7 weeks). The PE70 skirt induced corneal melting around the prosthesis.
Conclusion: Polypropylene encourages fibroblast ingrowth and shows good biological stability when used as a skirt material in S-KPro.
Keywords: polypropylene, fibroplasia, Seoul-type keratoprosthesis, porous polymers
The most common problem associated with keratoprosthesis (KPro) implantation results from an inadequate integration between the peripheral edge of the device and the residual rim of the host cornea. From the time that Barber first examined cellular ingrowth into the carbon fibre Teflon composite Propoplast in interlamellar pockets,1 many different materials intended for use in a KPro have been evaluated as to their biocompatibility, especially with respect to fibroplasia.2–5
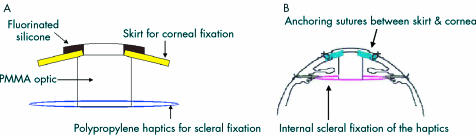
The Seoul-type keratoprosthesis (S-KPro) which is composed of a PMMA cylinder and a porous polymer skirt is being developed in our laboratory.6 A major characteristic of the S-KPro is a double fixed design, which includes the anchoring sutures of a skirt to the cornea and ab internal scleral fixation of the haptics, which synergistically improves the mechanical biostability (Fig 1). Therefore, the skirt, which is anchored to the cornea, does not need to serve as a strong support. An adequate coaptation with the surrounding cornea is emphasised in our model rather than a role as a support. We are currently attempting to find a material that enhances fibroblast migration even though it may have low tensile strength. Initially, polyurethane was used for the S-KPro skirt, because the fabrication of its pore size is easily controlled to allow fibroblast invasion.7 However, the polyurethane skirt was found to degenerate in a keratoprosthetic human eye after a long period. Therefore, we tried to find another porous material, which is biologically stable and allows more fibroblast invasion than polyurethane. Our goal was to compare the biological response, especially the fibroblast penetration and subsequent collagen deposition, using several polymers, including polyurethane, that are employed in the skirt of S-KPro in vivo experiments.
Figure 1.
(A) Schematic design of the S-KPro. (B) Schematic illustration showed the double fixed method. It includes suturing of a skirt to the cornea and fixation of the haptics to the sclera.
MATERIALS AND METHODS
Characteristics of materials
Four porous polymers intended for use as the peripheral skirt of a S-KPro were evaluated. These were a non-woven polypropylene (Q 2030 NW, Seon-Kyoung Company, Seoul, Korea) having a basis weight of 30.0 g/m2, and a thickness of 0.23 mm; a non-woven polyethylene terephthalate (PE70; C307NW, Seon-Kyoung Company, Seoul, Korea) having a basis weight of 70.0 g/m2, and a thickness of 0.25 mm; a non-woven polyethylene terephthalate (PE50; K205NW, Seon-Kyoung Company, Seoul, Korea) having a basis weight of 50.0 g/m2, and a thickness of 0.22 mm; a polyurethane (Biomaterials Research Center, Korea Institute of Science and Technology, Seoul, Korea) whose pore size was 40 μm and a porosity which ranged from 60% to 80%, having a thickness of 0.3 mm, which was made by a solvent casting particle leaching technique.7 The basis weight of the polyurethane ranged from 60.0 to 120.0 g/m2, according to the extent of the porosity.
The polymer was cut into 6 mm diameter discs for corneal stromal insertion. It was also used for the skirt of S-KPro. To examine a given pore size, materials were fixed in 2.5% glutaraldehyde, washed with phosphate buffered saline (PBS) buffer 0.1 mol/l and then dehydrated in a graded series of ethanols, then critical point dried in liquid carbon dioxide, coated with gold, and finally examined under the scanning electron microscopy (SEM, BS-130-C, Akashi Beam Technology, Japan).
Placement of the discs
All procedures conformed to the principles embodied in the ARVO statement for the use of animals in ophthalmic and vision research. The discs were inserted into each right eye of New Zealand white rabbits weighing 2.0–2.5 kg. The rabbits had been anaesthetised with an intramuscular injection of ketamine hydrochloride (30 mg/kg and Xylazine 5 mg/kg) and with a topical oxybuprocaine (proparacaine) hydrochloride. A 6 mm long and a half depth horizontal incision was made in the clear cornea, 1 mm away from the superior limbus. After an intralamellar corneal pocket was formed, the disc was then inserted into the pocket. The wound was then closed by a single 10-0 nylon suture, and topical oxytetracycline hydrochloride with polymyxin B sulphate ointments was applied. Postoperatively, no additional antibiotics or corticosteroids were administered. Five or more discs of each polymer were inserted for 2 and 5 month follow up experiments.
Seoul-type keratoprosthesis (S-KPro) implantation
At least, four S-Kpros were implanted for each type of polymer. The S-KPro implantation was performed using the technique described previously.6 Topical dexamethasone with polymyxin B sulphate and neomycin sulphate and oxytetracycline hydrochloride with polymyxin B sulphate ointments were administered postoperatively twice a day for a month.
Clinical evaluation
A clinical examination was performed biweekly using slit lamp biomicroscopy. Corneal oedema, neovascularisation, and granulomatous reaction were evaluated in the disc inserted rabbits. The degree of severity of each finding was graded subjectively. Corneal oedema with visible iris detail was defined as being grade +1, and severe corneal oedema that obscured iris detail as being grade +2. A new vessel reaching the disc margin was grade +1, a new vessel invading the half of the disc was +2, a new vessel invading three quarters of the disc was graded as +3, a new vessel invading the entire disc was graded as +4. Anterior flap melting and the retention period were examined in the S-KPro implanted eyes.
Light microscopy
A rabbit was sacrificed to examine fibroplasia with each type of material at 2 and 5 months. Disc inserted corneas were excised and fixed in 4% formaldehyde. Two μm sections were stained with haematoxylin and eosin and Masson-Trichrome. Frozen sections were cut from one third of the cornea for immunohistochemical experiments, dehydrated in a series of graded ethanols, cleared in xylene, and embedded in paraffin. Each cornea was sectioned at 4–6 μm, mounted on poly-l-lysine coated glass slides, and then air dried overnight at room temperature. The sections were dewaxed, taken through a series of graded ethanols, and then immersed in methanol containing 0.5% hydrogen peroxide to block endogenous peroxidase activity. They were subsequently immersed in water and were treated with immunostaining using the ABC method (Dako, Denmark) with primary vimentin incubations for 1 hour or 18 hours (that is, overnight) in a range of dilutions. Diaminobenzidine hydrogen peroxide was employed as the chromogen, and a methyl green counterstain was used. To determine whether keratocytes had migrated into the porous disc, a cross section was analysed.
The mean cell count was obtained from an examination of 10 high power fields (×400). In the Masson-Trichrome staining for collagen, absence of staining was defined to be grade 0, sparse staining was 1+, and dense staining was 2+. In the vimentin staining of fibroblast cytoplasm, and absence of staining was defined to be grade 0, a little staining was defined as 1+, and an intense staining was 2+.
Electron microscopy
The remaining corneas were treated by the same fixation and dehydration methods as stated previously6 and then they were examined by a transmission electron microscope (TEM; H-7100; Hitachi, Tokyo, Japan). An accumulation of collagen was observed in each sample.
RESULTS
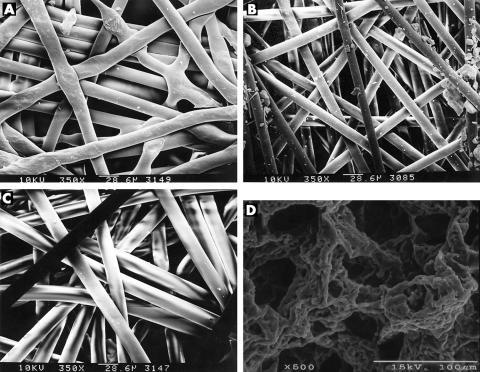
The SEM demonstrated that the pore diameter of all the given polymers was larger than 30 μm to allow fibroblast invasion (Fig 2).
Figure 2.
Scanning electron microscopy demonstrated that the pore diameter of all the given polymers was larger than 30 μm to allow fibroblast invasion. (A) Polyethylene terephthalate (PE70; C307NW), (B) polyethylene terephthalate (PE50; K205NW), (C) polypropylene (Q2030NW), (D) polyurethane.
In most of disc inserted rabbits, a corneal neovascularisation reaching the disc margin (+1) was observed 1 week postoperatively. From 3 weeks to 5 weeks, most of the discs were fully invaded by the new vessels (+4) (Table 1). Corneal oedema obscuring iris detail (+2) was found in most of the disc implanted eyes by the first weeks. It decreased markedly to +1 after 3 weeks and nearly cleared in most of the implanted eyes by 2 months. The corneal oedema tended to be less severe in polypropylene or PE70 disc implanted eyes than in PE50 or polyurethane disc inserted eyes (Table 1) . Two eyes, which had a polypropylene or a PE50 disc, had granulomatous reaction. No corneal necrosis or extrusion of the disc was found in disc embedded corneas.
Table 1.
Experimental results of the insertion of various polymers into the corneal stroma and the implantation of S-KPro with each polymer in rabbits
| Experimental material (commercial name) | ||||
| PP (Q2030NW) | PE 70 (C307NW) | PE 50 (K205NW) | PU | |
| Mean grade of vascularisation (3 weeks) | +3.5 | +2.5 | +3.75 | +4 |
| Mean grade of vascularisation (2 months) | +4 | +4 | +4 | +4 |
| Mean grade of corneal oedema (3 weeks) | +0.5 | +0.5 | +1 | +1 |
| Mean grade of corneal oedema (2 months) | +0.25 | +0 | +0.5 | +0.5 |
| Mean grade of corneal oedema (5 months) | 0 | 0 | 0 | 0 |
| Mean No of inflammatory cells (2 months)* | 55 (19.6) | 33 (13.1) | 28 (25.5) | 30.7 (5.8) |
| Mean No of fibroblasts (2 months)† | 46.4 (12.0) | 16 (5.3) | 34.2 (6.5) | 23.2 (13.4) |
| Grade of M-T staining (2 months) | 2+ | 1+ | 2+ | 1+ |
| Grade of vimentin staining (2 months) | 2+ | 1+ | 2+ | 1+ |
| Mean No of inflammatory cells (5 months) | 8.7 (1.9) | NA | 5.6 (2.6) | 8.4 (3.2) |
| Mean No of fibroblasts (5 months)‡ | 102.1 (27.9) | NA | 87.7 (22.2) | 65.4 (28.3) |
| Grade of M-T staining (5 months) | 2+ | NA | 2+ | 1+ |
| Grade of vimentin staining (5 months) | 2+ | NA | 2+ | 1+ |
| Mean retention time of S-KPro (weeks) | 20.7 | 18 | 14 | 15 |
| No of eyes with anterior flap melting after S-KPro implantation | 0 | 3 | 0 | 1 |
PP = polypropylene, PE70 = polyethylene terephthalate whose basis weight is 70 g/m2, PE50 = polyethylene terephthalate whose basis weight is 50 g/m2, PU = polyurethane, 3 weeks; 3 weeks after intrastromal insertion, 2 months = 2 months after intrastromal insertion, 5 months = 5 months after intrastromal insertion, No = number, M-T = Masson-Trichrome staining. Standard deviations given in parentheses
*It was statistically different between in polypropylene and in polyurethane (Mann-Whitney U test, p<0.05).
†It showed statistically significant increase in polypropylene and in PE50 compared with PE70 (Mann-Whitney U test, p<0.05).
‡It increased significantly in polypropylene and PE50 compared with polyurethane (Mann-Whitney U test, p<0.05).
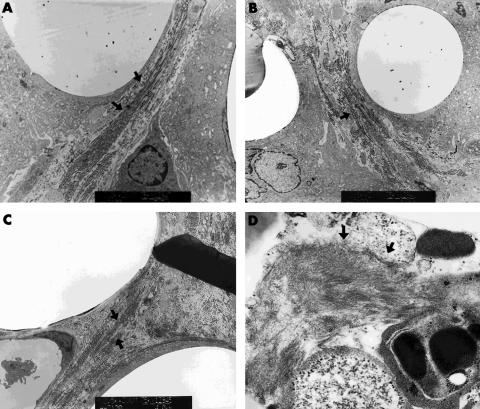
Two months after surgery, the mean number of inflammatory cells including giant cells, neutrophils, and eosinophils was 55 (SD 19.6), 28 (25.5), 33 (13.1), and 30.7 (5.8) in polypropylene, PE50, PE70, and polyurethane disc inserted eyes, respectively (Table 1). It was statistically different in polypropylene and in polyurethane (Mann-Whitney U test, p<0.05). The mean count of fibroblasts was 46.4 (SD12.0), 34.2 (6.5), 16 (5.3), and 23.2 (13.4) in polypropylene, PE50, PE70, and polyurethane disc inserted eyes, respectively (Table 1). It showed a statistically significant increase in polypropylene and PE50 compared with PE70 (Mann-Whitney U test, p<0.05). It was also larger in both polypropylene and PE50 than in polyurethane, although it was statistically insignificant. An intense vimentin staining was observed in polypropylene and PE50 disc implanted eyes (2+) compared to that in PE70 and polyurethane disc implanted eyes (1+) (Table 1). Dense collagen accumulation was detected in polypropylene and PE50 disc implanted eyes (2+) compared to that in PE70 and polyurethane disc implanted eyes (1+) using Masson-Trichrome staining (Table 1). Accumulated collagen was also demonstrated in polyethylene terephthalate and in polypropylene on TEM (Fig 3).
Figure 3.
Transmission electron microscope showed collagen accumulation 2 months postoperatively in the polymer that was implanted into the cornea. Black arrows indicate collagen deposition. (A) Polyethylene terephthalate (PE70; C307NW) (×8100), (B) polyethylene terephthalate (PE50; K205NW) (×5800), (C) polypropylene (Q2030NW) (×5800), (D) polyurethane (×4500).
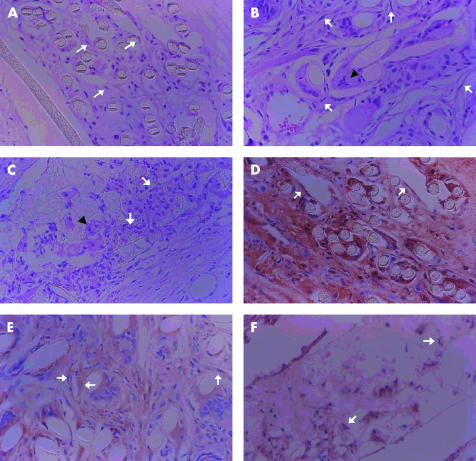
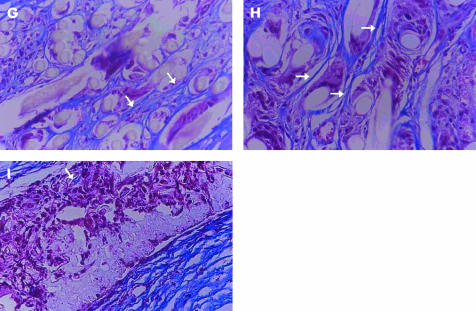
At 5 months postoperatively, we excluded PE70 from the experiment, because it was found to induce marginal necrosis when it was used as a skirt for the S-KPro. The number of inflammatory cells greatly decreased in all the eyes and most of the remaining cells were giant cells (histiocytes). The average number of inflammatory cells was 8.7 (SD1.9), 5.6 (2.6), and 8.4 (3.2) in polypropylene, PE50, and polyurethane disc inserted eyes, respectively, and they showed no statistical difference (p>0.05, Kruskal-Wallis test, Table 1). Fibroblast invasion increased markedly compared with the outcome at 2 months. The mean count of fibroblasts was 102.1 (SD27.9), 87.7 (22.2), and 65.4 (28.3) in polypropylene, PE50, and polyurethane disc inserted eyes, respectively (Table 1, Fig 4). It increased significantly in polypropylene and PE50 compared with polyurethane (Mann-Whitney U test, p<0.05). Polypropylene disc inserted eyes showed a remarkable increase in the number of fibroblasts. The staining patterns of collagen and vimentin were similar at 2 months (Table 1, Fig 4). TEM showed collagen accumulation similar at 2 months.
Figure 4.
Histological findings of the corneas after polymer insertion for fibroplasia and collagen accumulation 5 months postoperatively. (A–C) Haematoxylin and eosin staining (×400). Arrows indicate fibroblasts. The arrowheads indicate giant cells (histiocytes). (A) Polyethylene terephthalate (PE50; K205NW), (B) polypropylene (Q2030NW), (C) polyurethane. (D–F) Vimentin staining (×400). Arrows indicate a cytoplasmic stained fibroblast. (D) Polyethylene terephthalate (PE50; K205NW), (E) polypropylene (Q2030NW), (F) polyurethane. (G–I) Masson-Trichrome staining (×400). Arrows demonstrate collagen deposition. (G) Polyethylene terephthalate (PE50; K205NW), (H) polypropylene (Q2030NW), (I) polyurethane.
The mean retention period of the S-KPro was 18 weeks in PE70 S-KPro (n = 4), 14 weeks in PE50 S-KPro (n = 5), 20.7 weeks in polypropylene S-KPro (n = 5), and 15 weeks in polyurethane S-KPro (n = 4). Six rabbits had a well placed S-KPro, that remained over the 6 months after surgery. The skirts of the long lasting S-KPro comprised two polypropylene, two PE70, a PE50 and a polyurethane one. A polypropylene S-KPro was still in place at the time of writing, 15 months after surgery (Fig 5). No extrusion was found in any of the eyes. Most of these rabbits were sacrificed because of endophthalmitis, meningitis, or self limited disease. Three of PE70 S-KPro implanted eyes showed anterior corneal flap melting, which began after 4 weeks and then progressed to a totally melted level after 10–12 weeks. Only one rabbit suffered from partial corneal melting (25%) in the four polyurethane S-KPro implanted rabbits.
Figure 5.
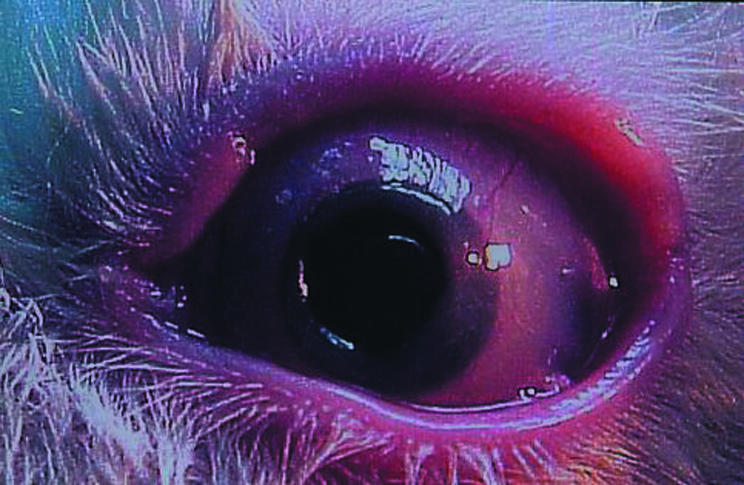
A well placed S-KPro made of a polypropylene skirt after 15 months.
DISCUSSION
The most common complications responsible for the failure of KPros are related to wound adaptation between the biological cornea and the non-biological KPro. The use of a porous material that encourages fibroblast penetration has been introduced to overcome these wound related problems. A fibrovascular ingrowth into the implant material has been demonstrated using Propoplasts,8 melt blown fibrous constructions of polybutylene/polypropylene,3,4 Dacron mesh,9,10 expanded polytetrafluoroethylene,2 polyurethane,11–13 and porous polyethylene.14 However, the ideal porous polymers still remain controversial.
The skirt of S-KPro was initially made from 40 μm diameter polyurethane.6 However, degradation of the polyurethane was found in keratoprosthetic human eyes after a long term follow up (data not shown). We tried to find a different non-degradable polymer. Ideally, this material should be pliable and elastic enough to avoid any mechanical stress, which may induce pressure necrosis at the points of contact. Therefore, a low tensile strength fabric, which is less likely to cause mechanical tension and non-woven fabrics to enhance fibroblast migration were considered to be candidates.
In terms of fibroblast invasion, the mean cell number at 2 months was larger in polypropylene and in PE50 than in polyurethane, although it was statistically insignificant. Moreover, the average cell count at 5 months increased significantly in polypropylene and PE50 compared with polyurethane. Taken together, they indicate that both polypropylene and PE50 has a greater capability of forming a fibroplasia than polyurethane. This may be because of the large pore size as a result of the non-woven meshwork. A more marked increase in fibroblast penetration was noted in the polypropylene disc compared with the PE50 disc, although it is statistically insignificant. Plentiful collagen accumulation was also noted in the polypropylene disc under TEM. Randall also demonstrated that extensive fibroplasia and collagen deposition occurred in a type of polybutylene/polypropylene material.3,4
Most of the polypropylene S-KPro discs showed a longer retention time without showing any corneal melting. In particular, a polypropylene S-KPro disc was stable up to 15 months. Together with data on fibroblast invasion, this suggests that polypropylene may be a promising material for use as a skirt for S-KPro.
Even though erosion did not occur around the edges of PE70 discs, partial necrosis of the cornea was observed around the margin of the skirt. It appeared to put much more torque pressure on the edge of the skirt than that of the disc, because the total diameter of the S-KPro is larger (10 mm) than that of the disc (6 mm), resulting in the development of corneal necrosis. This pressure necrosis was not observed in eyes with PE50 S-KPro and polypropylene S-KPro. PE70 is similar to PE50 but the outcome was somewhat different. Its basis weight was heavier (70 g/m2) than that of PE50 (50 g/m2). Consequently, it was too hard and stiff to induce anterior flap melting in the S-KPro implanted cornea. Additionally, the hardness and toughness of the PE70 made the cutting process for staining very difficult. In fact, part of a cornea was crushed during cutting because too much pressure was required to cut the implanted PE70 in the cornea. Possibly, it might cause the loss of the cells that existed between the fibres. Considering that the fibroblasts were loosely scattered between the fibres (as opposed to the inflammatory cells which adhered to the fibres) they could be more damaged during the cutting process. Therefore, it could explain why the number of fibroblasts in the PE70 discs was smaller than in PE50 discs. Accordingly, we concluded that PE70 was not suitable as a keratoprosthetic skirt.
Full vascularisation to an embedded disc and early corneal oedema, which reduced markedly after 3 weeks, were found in all eyes. As Barber mentioned that vascularised corneal beds were less likely to melt and allow extrusion,1 this finding was good for wound healing. However, considering that vessels are associated with recruitment of circulating inflammatory cells, ingrowth of the vessel may accelerate the influx of inflammatory cells. In fact, the acute phase of inflammation appears to be a natural phenomenon as a wound healing process against foreign bodies.15 When a foreign material comes into the body, an acute inflammatory response is initiated, which is accompanied by alterations in the permeability of vessels, exudation of plasma proteins, and the emigration of leucocytes after release of chemical mediators.15 This stage is then followed by a chronic granulation stage, which mainly consists of histiocytes, to establish a scar. It does not appear to be material specific.
Although the number of inflammatory cells in the polypropylene inserted corneas was greater than that in the polyurethane inserted corneas 2 months postoperatively, it reduced to a similar level to that in other polymer inserted corneas after 5 months. Histologically, acute destructive inflammation seemed to decrease in all the materials within 5 months. Finally, it might be impossible to prevent ingrowing new vessels and acute inflammation, irrespective of the material used. Consequently, initial treatment with anti-inflammatory drugs or amniotic membrane should be more aimed at reducing acute inflammation.
One problem associated with a non-woven fabric is that it can be untangled if it is exposed to mechanical strain caused by the eyelid. Pretreatments to enhance stability of the fibres is currently being investigated in our laboratory.
Additionally, rabbits are not regarded as the best model for corneal research since they have exceptional regenerative capabilities as regards epithelium, stroma, and endothelium.16 Even though this study has some limits in extrapolating human wound healing directly from rabbit wound healing with polymer, nevertheless, it is still useful to evaluate the general response with each material and to compare the materials with each other.
In conclusion, our results show that polypropylene (Q2030NW) encourages fibroblast ingrowth and collagen deposition, and shows biological stability when used as a skirt material in S-KPro. We believe this study provides an indispensable step in developing a biocompatible S-KPro.
Acknowledgments
Supported by a grant (HMP-98-G-2–048-a) from the HAN (Highly Advanced National) Project, Ministry of Health and Welfare and also supported in part by year 2000 BK21 project for medicine, dentistry, and pharmacy, Seoul, Korea.
REFERENCES
- 1.Barber J. Keratoprosthesis: past and present. Int Ophthalmol Clin 1987;28:103–9. [DOI] [PubMed] [Google Scholar]
- 2.Legeais JM, Renard G, Parel JM, et al. Expanded fluorocarbon for keratoprosthesis cellular ingrowth and transparency. Exp Eye Res 1994;58:41–52. [DOI] [PubMed] [Google Scholar]
- 3.Trinkaus-Randall V, Capecchi J, Leibowitz HM, et al. In vitro evaluation of fibroplasia in a porous polymer. Invest Ophthalmol Vis Sci 1990;31:1321–6. [PubMed] [Google Scholar]
- 4.Trinkaus-Randall V, Banwatt R, Capecchi J, et al. In vivo fibroplasia of a porous polymer in the cornea. Invest Ophthalmol Vis Sci 1991;32:3245–51. [PubMed] [Google Scholar]
- 5.Wu XY, Tsuk A, Leibowitz HM, et al. In vivo comparison of three different porous materials intended for use in a keratoprosthesis. Br J Ophthalmol 1998;82:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Wee WR, Chung ES, et al. Development of a newly designed double-fixed Seoul-type keratoprosthesis. Arch Ophthalmol 2000;118:1673–8. [DOI] [PubMed] [Google Scholar]
- 7.Mikos AG, Thorsen AJ, Czervonka LA, et al. Preparation and characterization of poly(L-lactic acid) foams. Polymer 1994;35:1068–1077. [Google Scholar]
- 8.Barber JC, Feaster FT, Priour DJ. The acceptance of a vitreous carbon alloplastic material, Propoplast, into the rabbit eye. Invest Ophthalmol Vis Sci 1980;19:182–90. [PubMed] [Google Scholar]
- 9.Kaiser D. Alloplastic replacement of canine trachea with Dacron. Thorac Cardiovasc Surg 1985;33:239–43. [DOI] [PubMed] [Google Scholar]
- 10.Pintucci S, Pintucci F, Cecconi M, et al. New dacron tissue colonisable keratoprosthesis: clinical experience. Br J Ophthalmol 1995;79:825–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi K, Nakayama Y, Matsuda T. Novel compliant and tissue permeable microporous polyurethane vascular prosthesis fabricated using excimer laser ablation. J Biomed Mater Res 1996;31:27–33. [DOI] [PubMed] [Google Scholar]
- 12.Kiremitci M, Pulat M, Senvar C, et al. Structural and cellular characterization of solvent-casted polyurethane membranes. Clin Mater 1990;6:227–37. [DOI] [PubMed] [Google Scholar]
- 13.Leidner J, Wong EWC, MacGregor DC, et al. A novel process for the manufacturing of porous grafts: Process description and product evaluation. J Biomed Mater Res 1983;17:229–47. [DOI] [PubMed] [Google Scholar]
- 14.Dreikorn K, Lebelenz J, Horsch R, et al. Alloplastic replacement of the partially resected canine urethra by expanded polytetrafluoroethylenegrafts. Urol Res 1979;7:19–21. [DOI] [PubMed] [Google Scholar]
- 15.Vijayasekaran S, Fitton JH, Hicks CR, et al. Cell viability and inflammatory response in hydrogel sponges implanted in the rabbit cornea. Biomaterials 1998;19:2255–67. [DOI] [PubMed] [Google Scholar]
- 16.Cintron C, Kubin CL. Regeneration of corneal tissue. Dev Biol 1977;61:346–57. [DOI] [PubMed] [Google Scholar]