Abstract
Background/aims: The effectiveness of occlusion therapy for the treatment of amblyopia is a research priority. The authors describe the design of the Monitored Occlusion Treatment for Amblyopia Study (MOTAS) and its methodology. MOTAS will determine the dose-response relation for occlusion therapy as a function of age and category of amblyopia.
Methods: Subjects progress through up to three study phases: (1) Assessment and baseline phase: On confirmation of eligibility, and after parental consent, baseline visual functions are determined, and spectacles prescribed as necessary; (2) Refractive adaptation phase: Subjects wear spectacles full time and return to clinic at 6 weekly intervals until 18 weeks, by which time all improvement due to refractive correction is complete; (3) Occlusion phase: All subjects are prescribed 6 hours of occlusion per day. Daily occlusion is objectively monitored using an occlusion dose monitor (ODM). Outcome variables: visual acuity (logMAR charts), log contrast sensitivity (Pelli-Robson chart), and stereoacuity (Frisby) are assessed at 2 weekly intervals until gains in visual acuity cease to be statistically verifiable.
Conclusion: Four methodological issues have been addressed; firstly, baseline stability of visual function; secondly, differentiation of refractive adaptation from occlusion; thirdly, objective measurement of occlusion dose and concordance; fourthly, use of validated outcome measures.
Keywords: amblyopia, occlusion therapy, occlusion dose monitor, refractive adaptation, dose-response
A mblyopia is the commonest cause of visual morbidity in childhood with a prevalence of 1–5%.1 Personal experience would suggest that it accounts for around nine out of 10 children’s eye appointments in the United Kingdom. This developmental anomaly of vision is characterised by a loss of spatial vision (usually unilateral) in the presence of strabismus, refractive error (bilateral ametropia or anisometropia), and/or form deprivation2 and is not immediately alleviated by refractive correction.
The most common treatment for unilateral amblyopia is occlusion (“occlusion therapy”) of the dominant eye with an opaque patch to promote visual function in the amblyopic eye. Occlusion is not a single prescriptive entity as the prescribed dose can vary from a few minutes a day to full time occlusion (all waking hours). Hiscox et al studied a group of 342 children with amblyopia, reporting that 48% of patients were prescribed 200–500 hours, and 28% over 500 hours of occlusion.3 While occlusion has been the mainstay of treatment for 250 years the evidence for its effectiveness has been questioned in a report published by the NHS Centre for Reviews and Dissemination,4 which concluded that “ . . .there do not appear to be any methodologically sound trials of the effect of treatment of amblyopia on visual function.” Studies that have attempted to determine the effectiveness of treatment have suffered from a variety of methodological constraints to which variations in the reported success rates of 19–93% can be attributed.3,5–11 The key issues that any methodologically sound effectiveness trial needs to address are establishment of an objective record of treatment; selection of appropriate outcome measurements; a sound definition of successful treatment outcome.
There are three principal components to amblyopia management: assessment, refractive correction (in many), and occlusion (or other specific treatments). Determining how each of these contributes to treatment outcome has not been attempted in any previous study.
Assessment and baseline measurements
To validate changes in visual function during treatment, accurate and stable pre-interventional baseline measurements are required. Subjectively measured visual functions, even where measured under identical conditions, may fluctuate with time, independent of any change in disease severity. In children, studies of repeatability12–14 have shown that changes in logMAR acuity may need to exceed 0.2 log units before one can conclude that a “real” change in performance has occurred.
Refractive correction
There have been occasional reports of improvement of vision in the amblyopic eye following a period of refractive correction particularly for those with anisometropic amblyopia,15,16 but until recently17 neither the magnitude nor the time course of this phenomenon was known. Hitherto, no clinical studies of amblyopia therapy have included a period of refractive adaptation within their design. Inevitably, when a refractive correction is prescribed more or less simultaneously with occlusion (as is commonly the case in clinical practice) it is impossible to differentiate their relative contributions from the eventual outcome.18
Amblyopia associated with ametropia, even in conjunction with strabismus, has been shown to be ameliorated from between 0.1 to 0.5 logMAR during a period of up to 23 weeks of refractive correction, although gains beyond 18 weeks did not exceed 0.1 log units of that already attained.17 Thus, in order that gains in visual performance are not falsely attributed to the prescribed occlusion, refractive adaptation should be completed before occlusion is begun.
Occlusion
Determination of the effectiveness of occlusion requires that the amount of treatment (occlusion dose) be measured objectively. Concordance with occlusion is problematic because of a range of factors including skin irritation, forced use of an eye with degraded vision, poor cosmesis, and lengthy treatment periods. A recent report19 has shown that the stress suffered by both parent and child during patching makes concordance with the treatment difficult to achieve. Consequently, on average, recorded occlusion is often only half that of the prescribed dose.19,20 Devices are now available to measure concordance known generically as occlusion dose monitors (ODMs).21–23 The ODM developed in our laboratory consists of an eye patch with two small electrodes attached to its under surface connected to a battery powered data logger by a thin lead (Fig 1). This has proved to be acceptable to children and their parents and provides an objective measure of the occlusion dose received by children undergoing routine treatment.24
Figure 1.
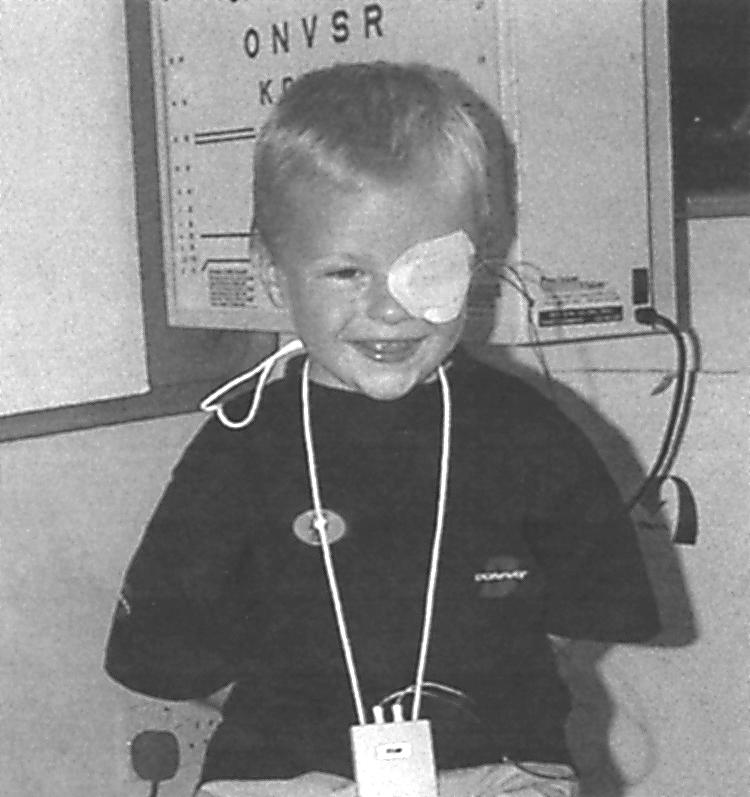
Child with occlusion dose monitor (ODM).
Trial methodology
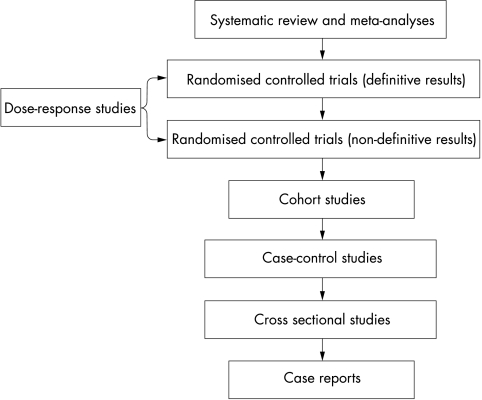
The process of establishing the effectiveness of a particular therapy involves the accumulation of evidence obtained from primary sources (that is, investigational) and ultimately by combining and critiquing this evidence into meta-analyses and systematic reviews. Evidence based medicine assigns a relative weighting to the various categories of evidence in the form of a hierarchy shown in Figure 2, with case histories providing the weakest, and meta-analyses and systematic reviews the most powerful, evidence for treatment effectiveness.25 Certain commentators have pointed out that claims for the effectiveness of occlusion therapy have only been gleaned from study designs capable of yielding evidence of a relatively low grade, and they have argued the case for a randomised controlled trial (RCT) of treatment effectiveness.4 In such a study design, patients are randomly allocated to receive one or more clinical interventions, which may include a placebo, or rarely, no treatment. Randomised controlled trials are judged to yield the highest grade of empirical evidence for or against the effectiveness of a given treatment.26 However, a prerequisite for undertaking an RCT in any treatment domain is some knowledge of the dose-response function—that is, in the present case, the amount of occlusion which, on average, results in a quantifiable gain in visual function. This objective is the primary goal of the MOTAS study design described in this paper. Implicit within this goal is an acknowledgement that were occlusion therapy shown not to result in significant improvement in visual outcome then no dose-response function would be obtained.
Figure 2.
The traditional hierarchy of evidence (modified from Greenhalgh24).
MOTAS STUDY DESIGN
Objectives
The objectives of MOTAS are stated in Table 1.
Table 1.
Aims and objectives of the MOTAS study
| Overall aim | |
| To determine the dose-response relation for occlusion therapy as a function of age and category of amblyopia | |
| Specific objectives | |
| 1 | To monitor objectively occlusion dose longitudinally throughout occlusion treatment |
| 2 | To record changes in visual performance longitudinally while children with amblyopia undergo occlusion therapy |
| 3 | To incorporate (1) and (2) above into a dose-response model of occlusion treatment and visual outcome as a function of age and category of amblyopia |
| 4 | To derive from the dose-response model, candidate occlusion regimens suitable for inclusion within randomised controlled trials of treatment effectiveness |
Ethical approval
Ethical approval was sought and obtained from two local research ethics committees.
Patient selection
Inclusion criteria and rationale are provided in Table 2.
Table 2.
Inclusion criteria and rationale for patient selection
| Criteria | Comment |
| Children aged 3–7 years | Accurate measurements of letter based visual acuity are unattainable in 50% of children under the age of 3 years but attainable in 75% of children 3.25 years of age.28 It is important that measurements are consistent and accurate, thus children under the age of 3 years are excluded from the study. Children over 7 years of age are not included as they fall outside the accepted sensitive period for visual development |
| Visual acuity of 0.1 log units or lower in worst eye and/or interocular difference of at least 0.1 log units | Visual acuity of 0.1 log units or worse is considered to be abnormal in this age group |
| Presence of anisometropia and/or strabismus | This group forms the majority of the population of children with amblyopia29 |
| No other ocular pathology | Form deprivation amblyopia is excluded because this forms a clinical entity distinct from the general population with amblyopia |
| No previous occlusion treatment | Dose-response functions in previously treated children may differ from those in whom treatment has already been undertaken |
| Knowledge of previous spectacle history | All subjects undergo full adaptation to their spectacles before entering the occlusion phase of the study. If children enter the study having already been prescribed spectacles it is important to establish that full spectacle adaptation has occurred |
| No learning difficulties | To obtain accurate and consistent measurements of visual function from every subject it is necessary to exclude patients with learning difficulties |
| Parental/guardian consent | In accordance with local ethics committee requirements |
Study phases
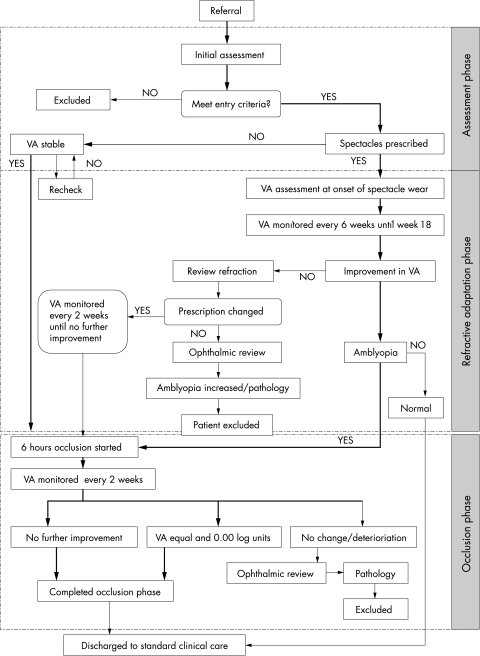
This prospective study comprises three phases: “assessment and baseline,” “refractive adaptation,” and “occlusion.” A flow chart identifying the path followed by subjects as they progress through the trial is shown in Figure 3. Parents of children eligible for inclusion in MOTAS are provided with an information sheet and given an opportunity to discuss the study with an investigator; written parental consent is a prerequisite of enrolment.
Figure 3.
An organisation flow chart indicating the path followed by subjects as they progress through the study. Typical progress of child is indicated by bold arrow.
Assessment and baseline phase
To ensure that stable baseline visual acuity measurements are achieved, visual performance is assessed before recruitment and again at study entry. If a significant change in visual acuity (defined as greater than plus or minus 0.20 log units) is observed between the recruitment assessment and the initial assessment, then a further measurement is sought. Having established baseline visual function the child then enters the next phase: refractive adaptation for the child requiring spectacle correction, or directly into the occlusion phase for the child not requiring refractive correction. Refractive error is assessed at the initial assessment by the author ARF with cycloplegic retinoscopy. The criteria used to determine the prescription of refractive corrections are provided in Table 3.
Table 3.
Criteria for clinically significant refractive error
| Bilateral hypermetropia | ≥1.50Ds |
| Bilateral myopia | ≥1.50Ds |
| Bilateral astigmatism | ≥0.75Dc |
| All astigmatism in combination with hypermetropia | |
| Anisometropia | ≥1.00Ds |
| Full astigmatic, anisometropic myopic and mild/moderate hypermetropic (+2.00Ds to +5.00Ds) refractive errors will be prescribed. Prescriptions for high hypermetropes are given within 2.00Ds of the full correction. | |
Refractive adaptation phase
This begins approximately 14 days after the initial assessment and determination of stable baseline measures (allowing for delivery of spectacles, where prescribed). Visual performance is recorded on this occasion without and with refractive correction, the latter being worn for the first time during this assessment. Subjects are instructed to wear spectacles (where prescribed) full time and are scheduled to return to clinic at 6 weekly intervals until 18 weeks of refractive adaptation is completed. Refractive adaptation is an essential component of amblyopia treatment; potentially eliminating the need for occlusion in some cases and reducing the amount required in others. In addition, the improved acuity seen at the completion of adaptation means that subsequent occlusion will probably be better tolerated than had it begun earlier when vision was worse.
Occlusion phase
Subjects remaining eligible (that is, meet the study’s operational definition of amblyopia, Table 2) are prescribed 6 hours of occlusion per day; a dose chosen on the following grounds. Firstly, this regimen constitutes a substantial amount of occlusion typical of that favoured by UK orthoptists27 generating results of direct relevance to clinical practice. Secondly, pilot studies have shown that much lesser doses of occlusion (such as 1 hour/day) fail to produce a clinically or statistically significant improvement in vision.24 Thirdly, on the understanding that prescribed and received dose are rarely the same, the adopted regimen allows for overconcordance as well as underconcordance and will facilitate the development of a dose-response model. Both visual function and monitored occlusion dose are recorded at 2 weekly intervals until acuity ceases to improve (defined as slope of acuity versus time plot not significantly different from zero). On completion of the occlusion phase, subjects are returned to standard care according to their clinical status.
Methods
Assessment of visual function
Visual function is assessed using distance log based charts for visual acuity and letter contrast sensitivity (Pelli-Robson). Stereoacuity is measured using the Frisby test. Three logMAR visual acuity charts are employed depending on subject age and ability: logMAR chart,30 crowded, and single logMAR.31 LogMAR tests conform to a regular geometric progression, have equal numbers of letters on each line, and use letters of near equal legibility and so permit interpolated scores. As each acuity test differs in its construction, visual acuity is measured with the same test throughout the study period to ensure continuity. If a child is able to progress to a more difficult test, this test will be included as an addition to the initial test battery. The most important outcome measure is the visual acuity of the amblyopic eye and this is recorded first at the start of every visit in case the child becomes fatigued. Visual acuity is measured without, and then with, spectacles (where appropriate), at the beginning and end of the final phase. Uncorrected visual function provides an indication of the overall effect of amblyopia treatment.
Objective monitoring of occlusion
Occlusion dose is monitored using an ODM as described above. At the start of the occlusion phase, the investigator explains in detail to the parents (and child where possible) the practicalities of wearing the monitor and adapted occlusion patches. By the end of the session parents will have gained familiarity with changing the patch and the child should be comfortable with wearing the monitor (suspended around the neck or attached to a belt clip). The parents are given an explanation of the ODM’s function and of the practice of occlusion therapy. At each subsequent visit, data from the ODM are downloaded to a PC and parents are given the opportunity to review the record of occlusion.
Statistical considerations
The dose-response function relating the objectively monitored occlusion dose with recorded visual performance is the crucial relation examined in this study. We require inference via a statistical model based on the fit of a multivariate parametric functional relationship of the form:
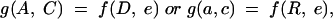 |
where A and C are the response variables visual acuity and contrast sensitivity, D and R are the input variables: total accumulated dose and daily dose, e is the random variability in the measurement of A and C, g is a vector function of the input variables and random error. (See Chuang-Stein and Agresti32 for a general discussion.)
The model will provide a quantitative perspective on how occlusion influences visual performance, and can subsequently be “interrogated” to provide answers to questions of interest:
To what extent does the occlusion dose influence outcome?
Can treatment be shortened and outcome improved as a consequence of achieving some threshold level of occlusion dose?
Is there a minimum dose, which can induce a therapeutic response?
Statistical analysis via Bayesian and classic parametric linear and non-linear modelling (utilising, for example, analysis of variance and model validation procedures) will be carried out. Additional analysis will consider the treatment response to the variables: (i) type of amblyopia; (ii) age of subject, and (iii) initial visual performance.
Sample size considerations
Lacking detailed previous information of the dose-response relation, a formal sample size calculation cannot be practically undertaken. For a proxy calculation, we can consider the detection of clinically significant difference between the beginning and end of occlusion in a homogeneous cohort (matched for age and initial severity) of patients. A statistical power analysis based on pilot investigations undertaken suggests that a total of 100 subjects will be required to complete the study. This analysis is based on a required power (1 − β) of 90% and an α of 5% to determine a significant difference between the groups as described above.
DISCUSSION
Screening for amblyopia is recommended for all UK children before the age of 5 years.33 It is our impression that management of this condition accounts for around 90% of patient visits to children’s eye services, thus consuming considerable NHS resource. Yet, as highlighted in the introduction, there have been no methodologically sound trials to investigate the effectiveness of occlusion treatment.4 The present study has been designed to investigate the dose-response relation of occlusion treatment. To undertake this task requires: (i) accurate repeat measurement of subjects’ visual performance and of their daily occlusion dose received as opposed to that prescribed; (ii) the ability of the study design to discriminate between gains in visual performance attributable to occlusion and those due to refractive correction. This is the first time occlusion dose will have been objectively monitored simultaneously with longitudinal measurement of visual function throughout an entire course of treatment. Until recently22 this work was not possible as there was no way of accurately monitoring treatment concordance and hence deriving a measure of treatment dose. (We have adopted here the term “concordance” in preference to the more common expression “compliance” to reflect current opinion on the need to promote a more egalitarian relation between patient and prescriber.34)
The empirical findings of MOTAS should inform those seeking to establish the effectiveness of occlusion therapy of suitable candidate regimens for inclusion in randomised controlled trials. Ultimately, better knowledge of the relation between treatment (total occlusion dose, occlusion/24 hour period) should enable the replacement of ad hoc prescribing by evidence based prescribing.
Acknowledgments
MOTAS is supported by the Guide Dogs for the Blind Association (GDBA).
REFERENCES
- 1.Attebo K, Mitchell P, Cumming R, et al. Prevalence and causes of amblyopia in an adult population. Ophthalmology 1998;105:154–9. [DOI] [PubMed] [Google Scholar]
- 2.Cuiffreda K, Levi D, Selenow A. Amblyopia: basic and clinical aspects. Oxford: Butterworth-Heinemann, 1991.
- 3.Hiscox FN, Strong N, Minshull C, et al. Occlusion for amblyopia: a comprehensive survey of outcome. Eye 1992;6:300–4. [DOI] [PubMed] [Google Scholar]
- 4.Snowden S, Stewart-Brown S. Pre-school vision screening: results of a systematic review. CRD Report 9. April 1997.
- 5.Watson PG, Sanac AS, Pickering MS. A comparison of various methods of treatment of amblyopia: a block study. Trans Ophthalmol Soc UK 1985;104:319–28. [PubMed] [Google Scholar]
- 6.Fulton AB, Mayer DL. Esotropic children with amblyopia: effects of patching on acuity. Graefes Arch Clin Exp Ophthalmol 1988;226:309–12. [DOI] [PubMed] [Google Scholar]
- 7.Bolger PG, Stewart-Brown S, Newcombe E, et al. Vision screening in preschool children: comparison of orthoptists and clinical medical officers as primary screeners. Br J Med 1991;303:1291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lithander J, Sjöstrand J. Anisometropic and strabismic amblyopia in the age group 2 years and above: a prospective study of the results of treatment. Br J Ophthalmol 1991;75:111–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epelbaum M, Milleret C, Buissenes P, et al. The sensitive period for strabismic amblyopia in humans. Ophthalmology 1993;100:325–7. [DOI] [PubMed] [Google Scholar]
- 10.Cleary M. Efficacy of occlusion for strabismic amblyopia: can an optimal duration be identified? Br J Ophthalmol 2000;84:572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mintz-Hitner HA, Fernandez KM. Successful amblyopia therapy initiated after age 7 years: compliance cures. Arch Ophthalmol 2000;118:1535–41. [DOI] [PubMed] [Google Scholar]
- 12.Kheterpal S, Jones HS, Moseley MJ, et al. Reliability of visual acuity in children with reduced vision. Ophthal Physiol Opt 1996;16:447–9. [PubMed] [Google Scholar]
- 13.Stewart CE. Comparison of Snellen and log-based acuity scores for school-aged children. Br Orthopt J 2000; 57:32–8. [Google Scholar]
- 14.McGraw PV, Winn B, Gray LS, et al. Improving the reliability of visual acuity measures in young children. Ophthal Physiol Opt 2001;3:173–84. [PubMed] [Google Scholar]
- 15.Kivlin JD, Flynn JT. Therapy of anisometropic amblyopia. J Paediatr Ophthalmol Strab 1981;18:47–56. [DOI] [PubMed] [Google Scholar]
- 16.Clarke WN, Noel LP. Prognostic indicators for avoiding occlusion therapy in anisometropic amblyopia. Am Orthopt J 1990;40:57–63. [Google Scholar]
- 17.Moseley MJ, Neufeld M, Fielder AR. Treatment of amblyopia by spectacles. Invest Ophthalmol Vis Sci 1998;39:S332. [Google Scholar]
- 18.Bishop JW. Treatment of amblyopia secondary to anisometropia. Br Orthopt J 1957;14:68–74. [Google Scholar]
- 19.Searle A, Vedhara K, Harrad R, et al. Compliance with eye patching in children and its psychosocial effects: a qualitative application of protection motivation theory. Psychol Health Med 2000;5:43–53. [Google Scholar]
- 20.Stewart CE, Fielder AR, Moseley MJ, et al. Is it all over in 6 weeks: Interim analysis of the Monitored Occlusion Treatment for Amblyopia Study (MOTAS). Invest Ophthalmol Vis Sci 2001;42:S399. [DOI] [PubMed] [Google Scholar]
- 21.Fielder AR, Auld R, Jones HS, et al. Compliance monitoring in amblyopia therapy. Lancet 1994;343:547. [DOI] [PubMed] [Google Scholar]
- 22.Fielder AR, Irwin M, Auld R, et al. Compliance monitoring in amblyopia therapy: objective monitoring of occlusion. Br J Ophthalmol 1995;79:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonsz HJ, Polling JR, Voorn R, et al. Electronic monitoring of treatment compliance in patching for amblyopia. Strabismus 1999;7:113–23. [DOI] [PubMed] [Google Scholar]
- 24.Moseley MJ, Fielder AR, Irwin M, et al. Effectiveness of occlusion therapy in ametropic amblyopia: a pilot study. Br J Ophthalmol 1997;81:956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenhalgh T. Papers that summarise other papers (systematic reviews and meta-analyses). Br Med J 1997;315:672–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Concato J, Shah N, Horwitz RI. Randomised controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan JHY, Thompson JR, Gottlob I. Differences in management of amblyopia between European countries. Invest Ophthalmol Vis Sci 2001;42:S398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egan DF, Brown R. Vision testing of young children in the age ranges 18 months to 4 years. Child Care Hlth Dev 1984;10:381–90. [DOI] [PubMed] [Google Scholar]
- 29.Shaw DE, Fielder AR, Minshill C, et al. Amblyopia—factors influencing age of presentation. Lancet 1988;293:207–9. [DOI] [PubMed] [Google Scholar]
- 30.Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am J Optom Physiol Opt 1976;53:740–5. [DOI] [PubMed] [Google Scholar]
- 31.McGraw PV, Winn B. Glasgow acuity cards: a new test for the measurement of letter acuity in children. Ophthal Physiol Opt 1993;13:400–3. [DOI] [PubMed] [Google Scholar]
- 32.Chuang-Stein C, Agresti A. Tutorial in biostatistics. A review of tests for detecting monotone dose-response relationship within ordinal response data. Stat Med 1997;16:2599–618. [DOI] [PubMed] [Google Scholar]
- 33.Blair M. The need for and the role of a coordinator in child health surveillance /promotion. Arch Dis Child 2001;84:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullen PD. Compliance becomes concordance. BMJ 1987;314:691–2. [DOI] [PMC free article] [PubMed] [Google Scholar]