Abstract
Aim: To evaluate the efficacy of oral cyclosporin A in the prevention and treatment of immune graft rejection in heavily vascularised, repeated keratoplasties with high risk for failure.
Methods: 21 consecutive patients with 28 repeated corneal transplants and four quadrant vascularised recipient bed were treated with oral cyclosporin A for an average period of 12 months (range 1–41 months) and followed for an average period of 26.6 months (range 6–106 months). The average cyclosporin A blood level was 325 ng/ml (range 180–421 ng/ml). Within this group of 21 patients, another 12 regrafts were not treated with cyclosporin A and served as a control group.
Results: Nine of the 28 regrafts (32%) treated with cyclosporin A remained clear. The Kaplan-Meier curve showed a constant decline in survival of the treated grafts, although the survival proportion during the first year of treatment was statistically higher for the treated group compared with the untreated group. Once immune regraft rejection occurred, the regraft failed despite treatment with cyclosporin A and extensive topical and systemic corticosteroids. Nine regrafts (32%) had immune graft rejection and all ultimately failed compared with five in the untreated regrafts (42%, p = NS). Ten other regrafts (36%) in the treatment group failed due to causes other than immune regraft rejection.
Conclusions: Systemic cyclosporin A has a limited beneficial effect in preventing immune graft rejection in repeated corneal transplants in a highly vascularised corneal bed. When immune graft rejection occurs in such regrafts, the prognosis is poor despite aggressive medical treatment. Causes other than immune regraft rejection may also result in poor visual outcome in patients with clear regrafts.
Keywords: cyclosporin A, corneal transplantation
Cyclosporin A is a T cell mediated immunosuppressant drug that has been successfully used as a topical or systemic preparation for ocular surface disorders such as vernal and atopic conjunctivitis,1,2 ocular pemphigoid,2 ulcerative keratitis,2 Mooren’s ulcer,2 dry eye,3,4 keratoconjunctivitis sicca,5 and uveitis.6,7
The effect of cyclosporin A on high rejection risk corneal transplants has not yet been fully established. Whereas cyclosporin A showed a substantial effect in prevention of immune graft rejection when given systemically in animal models,8–10 it may have a limited effect when administered topically to animals and humans.11,12 Several studies reported that topical cyclosporin A was effective in prevention of immune rejection of corneal graft in humans only when combined with topical corticosteroids.13–15 Topical cyclosporin A treatment has been reported to be beneficial in corneal transplanted eyes with no or minimal corneal vascularisation.16
Since the topical effect of cyclosporin A is controversial, and the effect of systemic treatment has not been evaluated in extensive corneal bed vascularisation, we evaluated the effect of oral cyclosporin A on corneal transplants with high risk for immune graft rejection and subsequent failure. We selected heavily vascularised, repeated corneal grafts to evaluate whether systemic cyclosporin A decreases the risk of immune rejection since, to the best of our knowledge this was never evaluated before. We assessed whether this treatment, combined with topical and systemic corticosteroids, results in timely suppression of immune rejection and increases graft survival. As an internal control, we employed the regrafts of this four quadrant vascularised, repeated transplantation group that were untreated with cyclosporin A to compare the immune rejection rate between treatment and no prophylactic treatment. We compared the survival of the cyclosporin treated group with the entire regrafted group during the same period.
METHODS
All patients who underwent repeated corneal transplantation between 1985 and 1998 and had four quadrant vascularisation of the corneal bed were included in the study. An informed consent specifying the potential benefit and adverse effects of oral cyclosporin A was obtained from all the patients before treatment.
Oral cyclosporin A (Sandimmun, Sandoz, Basle, Switzerland) was given daily starting immediately after surgery in a loading dose of 10 mg/kg/day. Thereafter, cyclosporin A blood levels were monitored and measured every 2 weeks to ascertain therapeutic levels of approximately 200–400 ng/ml. Blood pressure, complete blood cell count, serum creatinine and liver functions were evaluated every 2–4 weeks. All patients received topical corticosteroid every 2 hours, which was tapered gradually, over 2 months and antibiotic every 2 hours after surgery, which was discontinued after epithelialisation (2–3 weeks). Thereafter, treatment included oral cyclosporin A and a maintenance dose of topical corticosteroid twice daily. Patients were followed on a regular basis every day for the first postoperative week, weekly in the first month, and then monthly. The patients were instructed to undergo an immediate ophthalmological examination whenever they felt ocular pain or discomfort, decreased vision, redness of the eye, or following ocular trauma.
At each follow up visit, a complete ocular examination was performed. This included recording visual acuity with Snellen chart, applanation tonometry, anterior and posterior segment evaluation with a slit lamp, and indirect ophthalmoscopy. The corneal regraft was evaluated for clarity and signs of immune response such as epithelial and/or endothelial rejection line, corneal oedema, and anterior chamber inflammatory reaction. Corneal regraft failure was defined as irreversible graft oedema or vascularised scar. Secondary glaucoma was defined as intraocular pressure exceeding 21 mm Hg in repeated measurements.
When signs of immune graft rejection were identified, the patients were hospitalised and topical dexamethasone sodium phosphate 0.1% every hour combined with intravenous methylprednisolone 3 mg/kg/day for 3 days were immediately added followed by oral prednisolone acetate 1 mg/kg/day. The topical and systemic treatments with corticosteroids were tapered when immune graft rejection resolved or when the regraft failed. Cyclosporin A was given continuously for an average of 12 months (range 1–41 months) and the follow up continued for an average of 26 months (range 6–106 months). The minimal follow up period after the last repeated transplantation was 6 months. The cyclosporin A treatment was discontinued in the following settings: (A) failure of the corneal regraft, (B) irreversible visual losses due to causes other than regraft failure, (C) appearance of side effects. Otherwise, treatment was continued indefinitely. The patient remained in the study group when the cyclosporin A was withdrawn. The measured average blood level of cyclosporin A was 325 ng/ml (range 180–421 ng/ml throughout the study period).
Within the four quadrant vascularised, repeated keratoplasties, all regrafts untreated with systemic cyclosporin A served as a control group. All these regrafts were arbitrarily selected for immediate treatment with topical corticosteroid drops every 2 hours, which was tapered gradually, over 2 months and antibiotic drops every 2 hours after surgery, which was discontinued after epithelialisation (2–3 weeks). Thereafter, treatment included a maintenance dose of topical corticosteroid twice daily.
Another reference group for comparing the cumulative survival proportion (Kaplan-Meier curve) included all the patients that underwent repeated corneal transplantation during the study period from 1985 to 1998. In this group, 122 regrafts were performed in 86 eyes of 80 patients. The data for the survival analysis were available in 110 of the 122 repeated grafts.
Patients
Twenty one consecutive patients (23 eyes) with four quadrant vascularised recipient bed were included in the study. Of the 21 patients, 14 were male and seven were female. The average age at the onset of treatment was 52 years (range 16–77 years).
In the group of 23 eyes, treatment with systemic cyclosporin A was given for 28 regrafts with four quadrant vascularised recipient bed (Table 1). Five were treated for two subsequent repeated grafts and the rest for one repeated graft. Two patients were treated with oral cyclosporin A for repeated grafts in both eyes.
Table 1.
Summarised data of four quadrant repeated corneal transplanted patients treated and untreated with systemic cyclosporin A
| Parameter | Regrafts treated with cyclosporin A | Regrafts untreated with cyclosporin A |
| Number of regrafts | 28 | 12 |
| Follow up (months) | ||
| Average | 26 | 20 |
| Range | 6–106 | 2–65 |
| Cyclosporin A treatment (months) | ||
| Average | 12 | |
| Range | 1–41 | |
| Regraft survival (months) | ||
| Average | 16.1 | 10.9 |
| Range | 1–40 | 1–36 |
| Survival of regrafts complicated by immune rejection (months) | ||
| Average | 16.5 | 18.8 |
| Range | 10–40 | 3–36 |
| Survival of regrafts with other complications (months) | ||
| Average | 17.6 | 5.2 |
| Range | 1–39 | 1–11 |
| Number of failed regrafts | 19 (68%) | 11 (92%) |
| Number of failed regrafts due to immune rejection | 9 (32%) | 5 (42%) |
| Visual acuity at presentation | LP–20/100 | LP–20/100 |
| Final visual acuity | NLP–20/30 | LP–20/200 |
LP = light perception, NLP = no light perception.
In the group of the 21 patients with four quadrant vascularised regrafts, 12 regrafts that were not treated with systemic cyclosporin A served as a control group to evaluate the difference in the occurrence of immune regraft rejection between the groups and the influence of treatment on the resolution of the rejection. Statistical significance was calculated with κ2 test for categorical covariates and Fisher exact test for samples with expectancy of less than 5. A p value <0.05 was considered statistically significant.
All corneal grafts were harvested and kept in Optisol-GS medium (Chiron Ophthalmics, Irvine, CA, USA) in 4°C for mostly up to 4 days until transplantation. The donor corneas were not tissue matched for HLA and ABO compatibility.
The most common (12 of the 23 eyes) primary indication for corneal grafting was vascularised corneal scar of which four eyes had a history of herpetic keratitis, four eyes had traumatic scars, two eyes were following perforated corneal ulcer, and two were following chemical burn. In addition, five eyes had corneal dystrophies (Fuchs’ and congenital hereditary endothelial dystrophy), two had aphakic bullous keratopathy (ABK), two had pseudophakic bullous keratopathy (PBK), one had keratoconus, and one was with unknown aetiology. The indications for the repeated transplantation were irreversible immune rejection in nine cases and other various causes for graft failure in the remainders.
In 13 cases, systemic cyclosporin A was given to the first regraft. In 11 cases, cyclosporin A was given for the second regraft. In three cases, oral cyclosporin A was given for the third regraft and in one case for the fourth regraft.
Visual acuity before the primary keratoplasty was 20/100 in two eyes, 20/200 in five eyes, counting fingers at less than 20 feet in 12 eyes, hand movements in two eyes, and light perception in two eyes in the treated group.
RESULTS
Nine of the 28 cyclosporin A treated regrafts (32%) in 23 eyes (39%) remained clear (Table 1). Seven of them (25%) had improvement in visual acuity. Nine regrafts (32%) had endothelial immune graft rejection. In six of the rejected regrafts (66.6%), the immune graft rejection occurred during and despite the treatment with oral cyclosporin A. Three of them were rejected after discontinuation of the cyclosporin A. All the regrafts that had immune rejection ultimately failed despite continuation of treatment with cyclosporin A and adding topical and systemic corticosteroids. Ten other regrafts (36%) failed due to causes other than immune graft rejection. Some had several causes for failure. Cyclosporin A was withdrawn in these patients when the regraft failed, but they were included in the analysis.
Nine regrafted patients that were treated with cyclosporin A had a history of irreversible immune rejection of their primary graft. Of these, five were rejected and failed, two failed because of other causes (glaucoma and corneal surface disorders), and only two were successful. One, a 16 year old patient, had recurrent episodes of immune rejection in both transplanted eyes despite cyclosporin A treatment.
In five of 12 regrafts (42%) in the high failure risk regrafts untreated with cyclosporin A that served as a control group, immune regraft rejection occurred (Table 1). In all these five cases failure of the regraft occurred. This incidence was similar to the 32% of immune regraft rejection found in the high failure risk regrafts treated with cyclosporin A (p = NS, Fisher exact test).
Regraft rejection occurred after an average period of 10.5 months (range 5–24 months). The average survival period of the failed regrafts due to immune graft rejection was 16.5 months (range 10–40 months). The average survival period of the 10 regrafts that failed due to other causes was comparable (mean 17.6 months, range 1–39 months). The shortest survival period of the regrafts was in three cases with persistent epithelial defects (1–3 months), and the longest survival period was in two cases of pseudophakic bullous keratopathy and in five cases of secondary glaucoma (18–39 and 13–35 months respectively).
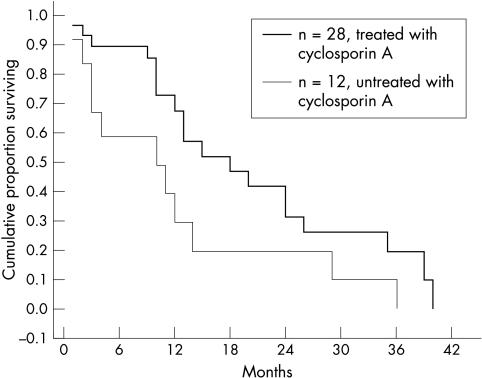
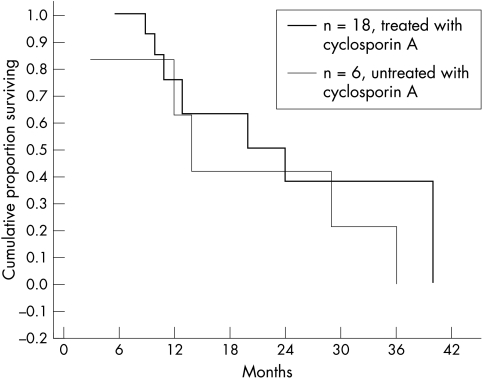
A Kaplan-Meier curve showed that the final cumulative survival was low and similar in the cyclosporin A treated and untreated groups (Fig 1). The cumulative survival proportions for 6, 12, 24, and 36 months were 0.89, 0.67, 0.31, and 0.19 for the treated group and 0.58, 0.29, 0.19, and 0.10 for the untreated group respectively. Statistically higher regraft survival proportions of the treated group were found for the first 6 and 12 postoperative months (p=0.039, 0.037, 0.297, 0.063 respectively, Fisher exact test). However, when a Kaplan-Meier curve was plotted for all regrafts excluding those that failed due to causes other than immune rejection, it was similar to both treated and untreated groups (Fig 2). In addition, all regrafts with immune rejection failed despite of treatment with systemic cyclosporin A and topical and systemic corticosteroids.
Figure 1.
Kaplan-Meier cumulative survival proportion of repeated corneal transplants treated and untreated with systemic cyclosporin A.
Figure 2.
Kaplan-Meier cumulative survival proportion of repeated corneal transplants the treated and untreated with systemic cyclosporin A excluding regrafts that failed due to causes other than immune rejection.
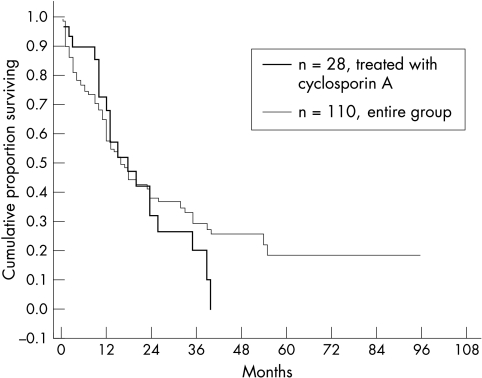
A Kaplan-Meier curve of the treated group showed a higher cumulative survival proportion compared with the entire repeated keratoplasties group during the first year of follow up, a comparable proportion during the second year and lower after the second year (Fig 3). However, the differences in the survival proportions were statistically insignificant. The cumulative survival proportions at 12, 24, and 36 months were 0.67, 0.31, and 0.19 for the treated group and 0.57, 0.38, and 0.29 for the entire regrafted group respectively (p = NS, κ2 test). In the entire regrafted group, 44 of the 86 eyes (51%) remained with clear regrafts compared with 39% in the cyclosporin A treated regrafted group (p = NS, κ2 test).
Figure 3.
Kaplan-Meier cumulative survival proportion of regrafts treated with systemic cyclosporin A versus the entire regrafted group.
The regrafts that failed due to immune graft rejection had a final visual acuity of 20/100 to no light perception. Three of these patients had visual acuity of light perception due to accompanied end stage secondary glaucoma, and one patient had no light perception due to inoperable retinal detachment. The five patients with regraft, which failed as a result of other causes, had visual acuity between 20/200 and light perception, and in two of them, the failure was due to secondary glaucoma. The patients with clear grafts had an average visual acuity of 20/80 (20/30–20/200). In two of these patients, visual acuity was 20/100 and 20/200 due to irregular astigmatism.
DISCUSSION
Repeated corneal transplantation is a subset of high risk condition for immune graft rejection. Repeated grafts usually have extensive vascularisation of the corneal bed preoperatively and a history of previous failure, sometimes due to immune graft rejection. As such, repeated corneal transplants are a suitable subgroup for studying the effect of systemic cyclosporin A on the survival of corneal grafts with high risk for immune rejection and failure and comparing it with untreated regrafts. The disadvantages of this study model are the heterogeneity of indications for the primary transplantation and the diverse causes for failure of the primary transplants. Nevertheless, studying the effect of systemic cyclosporin A is extremely important to evaluate its efficacy in prevention and reversibility of the immune regraft rejection.
We evaluated the effect of systemic cyclosporin A on repeated graft survival since the efficacy of topical cyclosporin A alone in animal and humans models is controversial.11,12,17,18 Williams et al11,12 showed that the effect of topical cyclosporin A was not superior to topical corticosteroids in rabbits and in inbred rats. In contrast with Hunter et al,17 Roussel18 found that topical cyclosporin A with or without dilution in arachis oil may prolong corneal graft survival. In evaluation of the effect of systemic treatment with cyclosporin A, Coster et al,8 and Hill and Maske9 showed prolonged graft survival in rabbits. We found a similar effect and showed a suppression of the immune corneal allograft rejection in heavily vascularised rabbit corneas, following alkali burn, as a model for high rejection risk10 and we expected a similar effect when given to humans.
The results of our study showed that oral cyclosporin A seems to have a limited benefit in the prevention of immune graft rejection in heavily vascularised, repeated keratoplasties in humans. Only 25% of the regrafts remained clear with significant improvement in visual acuity; 32% of the regrafts had immune graft rejection, and ultimately all of them failed despite the continuation of oral cyclosporin A and the addition of topical and systemic corticosteroids.
The Kaplan-Meier curve showed a delayed failure rate of the treated group compared with the untreated group during the first 12 postoperative months. This was also the average time of cyclosporin A treatment, suggesting that cyclosporin A might have had effect in prevention of immune regraft rejection and failure while being used. However, despite cyclosporin A treatment, when the regrafts that failed due to causes other than immune rejection were excluded, the survival was comparable during the entire follow up period for both the treated and untreated groups.
Our rate of regraft survival was higher than previously reported by some authors for non-high rejection risk regrafts without systemic cyclosporin A treatment.19,20 In our previous study,21 we found that 55% of eyes with repeated keratoplasties and no high rejection risk survived without cyclosporin A treatment. These findings were similar to those reported by Cowden et al22 who reported a 63% of success, though his follow up period was shorter (1 year). In addition, the survival of regrafts without four quadrant vascularisation was statistically higher than four quadrant vascularised regrafts treated with cyclosporin A (p<0.05, κ2 test).
Hill23,24 treated 18 patients with high risk keratoplasties with oral cyclosporin A in conjunction with topical and systemic corticosteroids and found that a higher percentage of 88.9% remained clear. However, in his study, only 15 of the 18 were repeated grafts. The degree of corneal vascularisation and indications for the primary transplantations and their preoperative and postoperative status were not reported. Therefore, a comparison of the results is of limited value.
The only two graft failures in Hill’s study occurred after discontinuation of cyclosporin A, while we found that 66.6% of the failed regrafts due to immune graft rejection had the rejection episode while under systemic cyclosporin A treatment. This suggests that prevention of immune graft rejection is limited in heavily vascularised, repeated keratoplasties. The beneficial effect of oral cyclosporin A in prevention of immune rejection, in Hill’s study, may also have been biased by its conjunction with systemic and topical treatment with corticosteroids.
We found that once the immune rejection occur the regraft fails despite continuation of systemic cyclosporin A and extensive topical and systemic corticosteroids, in contrast with Hill’s study, in which three grafts had resolution of the immune rejection. Failure was time dependent as demonstrated in Kaplan-Meier curve and therefore extended period of follow up is essential when evaluating the efficacy of drugs such as cyclosporin A. Thus, it seems that vascularised corneas behave like other vascularised organs, but because of their smaller size, they may be more affected by immune rejection limiting the effect of cyclosporin A.
We found that causes other than immune rejection had a major role in failure of repeated corneal grafts treated with systemic cyclosporin A. These included refractory secondary glaucoma, bullous keratopathy due to endothelial decompensation, ulcers, and persistent epithelial defects. Since they decreased regraft survival, they also diminished the validity of cyclosporin A treatment. Graft failure, owing to causes other than immune graft rejection, were not reported previously.24 In our study, failure due to secondary glaucoma and bullous keratopathy occurred rather late in comparison with the failure due to immune rejection or persistent epithelial defects and ulcers, again stressing the importance of an extended follow up period.
It should be stressed that repeated corneal grafts are more prone to develop postoperative complications compared with primary grafts21 and visual outcome of eyes with regrafts may be poor even in eyes with a clear graft due to other intraocular pathologies.
The potential significant adverse effects of systemic cyclosporin A include nephrotoxicity, hepatotoxicity, and bone marrow toxicity as well as systemic hypertension.25 These side effects are rare when the drug blood level is well monitored and kept at low therapeutic levels of approximately 200 ng/ml. We did not experience these significant side effects aside from mild hirsutism in three patients and gingival hypertrophy in two.
The limitations of our study are its retrospective nature and limited number of patients in this highly specified group that could be recruited. Although the literature advocates the use of cyclosporin A combined with corticosteroids in prevention and treatment of immune graft rejection, this advocation applies to grafts without significant vascularisation. Our study indicates that for highly vascularised, repeated keratoplasties, systemic cyclosporin A may have a limited protective effect against immune rejection, but when regraft rejection occurs, cyclosporin A is probably insufficient even in conjunction with corticosteroids to reverse it.
Acknowledgments
The authors thank Orly Yakir, MA, for the statistical analysis.
Presented in part at the 2001 Annual Meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, FL, USA, May, 2001
The authors do not have any financial or proprietary interest in any of the products mentioned in this paper.
REFERENCES
- 1.BenEzra D, Pe’er J, Brodsky M, et al. Cyclosporine eyedrops for the treatment of severe vernal conjunctivitis.Am J Ophthalmol 1986;101:278–82. [DOI] [PubMed] [Google Scholar]
- 2.Holland EJ, Olsen TW, Ketcham JM, et al. Topical cyclosporine-A in the treatment of anterior segment inflammatory disease.Cornea 1993;12:412–9. [DOI] [PubMed] [Google Scholar]
- 3.Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease.Ophthalmology 2000;107:631–9. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson DT, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate to severe dry eye disease: a dose-ranging, randomized trial. The cyclosporin A Phase 2 Study Group.Ophthalmology 2000;107:967–74. [DOI] [PubMed] [Google Scholar]
- 5.Laibovitz RA, Solch S, Adriano K, et al. Pilot trial of cyclosporine 1% ophthalmic ointment in the treatment of keratoconjunctivitis sicca.Cornea 1993;12:311–23. [DOI] [PubMed] [Google Scholar]
- 6.Nussenblatt RB, Palestine AG, Chan CC. Cyclosporin A therapy in the treatment of intraocular inflammatory disease resistant to systemic corticosteroids and cytotoxic agents.Am J Ophthalmol 1983;96:275–82. [DOI] [PubMed] [Google Scholar]
- 7.Nussenblatt RB, Palestine AG, Chan CC, et al. Effectiveness of cyclosporin therapy for Behçet disease.Arthritis Rheum 1985;28:671–9. [DOI] [PubMed] [Google Scholar]
- 8.Coster DJ, Shepard WFI, Chin Fook T, et al. Prolonged survival of corneal allografts in rabbits treated with cyclosporine A.Lancet 1979;2:688–9. [DOI] [PubMed] [Google Scholar]
- 9.Hill JC, Maske R. An animal model for corneal graft rejection in high risk keratoplasty.Transplantation 1988;46:26–30. [DOI] [PubMed] [Google Scholar]
- 10.Rehany U, Waisman M. Suppression of corneal allograft rejection by systemic cyclosporine-A in heavily vascularized rabbit corneas following alkali burn.Cornea 1994;13:447–53. [DOI] [PubMed] [Google Scholar]
- 11.Williams KA, Erickson SA, Coster DJ. Topical steroid, cyclosporin A, and the outcome of rat corneal allografts.Br J Ophthalmol 1987;71:239–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams KA, Grutzmacher RD, Roussell TJ, et al. A comparison of the effects of topical cyclosporin and topical steroid on rabbit corneal allograft rejection.Transplantation 1985;39:242–4. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann F, Wiederholt M. Lokale behandlung des hornhaut transplantates beim menscheu mit cyclosporin A.Klin Monatsbl Augenheilkd 1985;187:92–6. [DOI] [PubMed] [Google Scholar]
- 14.Goichot-Bonnat L, Chemla P, Pouliquen Y. Cyclosporine-A collyre dans la prevention du rejet de greffe de cornee a haut risque.J Fr Ophtalmol 1987;10:213–6. [PubMed] [Google Scholar]
- 15.Miller K, Huber C, Niederwieser D, et al. Successful engraftment of high-risk corneal allografts with short-term immunosuppression with cyclosporine.Transplantation 1988;45:651–3. [DOI] [PubMed] [Google Scholar]
- 16.Zhao JC, Jin XY. Local therapy of corneal allograft rejection with cyclosporine.Am J Ophthalmol 1995;119:189–94. [DOI] [PubMed] [Google Scholar]
- 17.Hunter PA, Wilhelmus KR, Rice NSC, et al. Cyclosporine A applied topically to the recipient eye inhibits corneal graft rejection.Clin Exp Immunol 1981;45:173–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Roussell TJ, Osato MS, Wilhelmus KR. Cyclosporine and experimental corneal transplantation.Transplant Proc 1983;15(Suppl):3081–3. [Google Scholar]
- 19.Robinson CH. Indications, complications and prognosis for repeat penetrating keratoplasty.Ophthalmic Surg 1979;10:27–34. [PubMed] [Google Scholar]
- 20.Insler MS, Pechous B. Visual results in repeat penetrating keratoplasty.Am J Ophthalmol 1986;102:371–5. [DOI] [PubMed] [Google Scholar]
- 21.Bersudsky V, Blum-Hareuveni T, Rehany U, et al. The profile of repeated corneal grafts.Ophthalmology 2001;108:461–9. [DOI] [PubMed] [Google Scholar]
- 22.Cowden J, Kaufman HE, Polack FM. The prognosis of keratoplasty after previous graft failures.Am J Ophthalmol 1974;78:723–5. [DOI] [PubMed] [Google Scholar]
- 23.Hill JC. The use of systemic cyclosporine A in human corneal transplantation. A preliminary report.Doc Ophthalmol 1986;62:337–40. [DOI] [PubMed] [Google Scholar]
- 24.Hill JC. The use of cyclosporine in high-risk keratoplasty.Am J Ophthalmol 1989;107:506–10. [DOI] [PubMed] [Google Scholar]
- 25.Nussenblatt RB, Palestine AG. Cyclosporine: immunology, pharmacology and therapeutic uses.Surv Ophthalmol 1986;31:159–69. [DOI] [PubMed] [Google Scholar]