Abstract
Aim: To investigate the correlation between tests of visual function and perceived visual ability recorded with a quality of life questionnaire for patients with uveitis.
Methods: 132 patients with various types of uveitis were studied. High (monocular and binocular) and low (binocular) contrast logMAR letter acuities were recorded using a Bailey-Lovie chart. Contrast sensitivity (binocular) was determined using a Pelli-Robson chart. Vision related quality of life was assessed using the Vision Specific Quality of Life (VQOL) questionnaire.
Results: VQOL declined with reduced performance on the following tests: binocular high contrast visual acuity (p = 0.0011), high contrast visual acuity of the better eye (p = 0.0012), contrast sensitivity (p = 0.005), binocular low contrast visual acuity (p = 0.0065), and high contrast visual acuity of the worse eye (p = 0.015). Stepwise multiple regression analysis revealed binocular high contrast visual acuity (p <0.01) to be the only visual function adequate to predict VQOL. The age of the patient was also significantly associated with perceived visual ability (p <0.001).
Conclusions: Binocular high contrast visual acuity is a good measure of how uveitis patients perform in real life situations. Vision quality of life is worst in younger patients with poor binocular visual acuity.
Keywords: uveitis, quality of life, visual function
Uveitis is a major cause of severe visual impairment.1 A report on 582 uveitis patients showed that 203 (35%) suffered from significant visual loss.1 Bilateral legal blindness developed in 22 (4%) of the patients, 26 (4.5%) had one blind eye with visual impairment of the other, and nine (1.5%) had bilateral visual impairment. Unilateral losses occurred in 146 (25%), blindness in 82 (14%), and visual impairment in 64 (11%) patients. The main cause of visual impairment was cystoid macular oedema.
The incidence of uveitis has been reported as 38 per 100 000 in a general population, and the annual incidence as 17 per 100 000, with a maximum incidence in the 25–44 year age group.2 Uveitis is not usually included in surveys of the causes of blindness and is, therefore, probably underestimated.3 Studies have estimated that uveitis accounts for 10–15% of all cases of total blindness in the United States.4 In the 1993 annual report of Research to Prevent Blindness, an estimated 2 300 000 Americans suffered visual impairment resulting from uveitis.5
Another study on 148 uveitis patients, which examined the incidence, cause, and duration of visual loss, showed that 86 patients (58.1%) had reduced visual acuity in at least one eye at some period during their disease.6 Of these 86 patients, 44 (51.1%) had reduced vision in only one eye and 42 (48.9%) had reduced vision in both eyes. Furthermore, 33 patients (38.3%) had reduced visual acuity of <20/200 in at least one eye. The median duration of visual loss was 19.5 months with the main causes being cystoid macular oedema (31.3%), cataract (20.9%), and a combination of these (24.4%).
These results indicate that visual loss is a frequent and recurring problem in uveitis patients. As uveitis mostly affects individuals of working age this visual impairment has important economic consequences with numerous days off work or job losses, and in the younger age group interferes with education. Socioeconomic factors, such as driving, social activities, and pastimes may also be affected.
Current methods of assessing visual loss are mainly based around visual acuity (VA) measurements; it is from this measurement that many clinical and surgical decisions are made. Visual acuity characterises the ability to use visual information at high spatial frequencies, but it does not reveal a possible deficit at low or intermediate spatial frequencies.
Since the “real world” is composed of objects of varying spatial frequencies and contrast, VA alone may be too simplistic an assessment for everyday visual tasks. Several studies suggest that high contrast VA, low contrast VA, and contrast sensitivity may together provide a more useful assessment of visual function.9–11
Patients’ own perception of their visual performance is important. A vision related quality of life questionnaire allows an assessment of what is important to patients with respect to their vision, thus capturing the full extent of the disability suffered by the patient in the real world. A number of vision targeted quality of life questionnaires exist, such as the VF-14, VF-25, and the VQOL.12–16
The aim of this study was to ascertain how the visual disabilities experienced by uveitis patients affects them in the “real world.” Visual performance, using a series of clinical visual function tests, was compared with patients’ perceived visual ability, recorded with a vision related quality of life questionnaire.
METHODS
Tests (see below) were carried out on patients with various types of uveitis attending the uveitis clinics of the Birmingham and Midland Eye Centre; the uveitis clinics are predominantly secondary and tertiary referral clinics. Over a 4 month period, previous attenders whose appointments for the morning clinic fell between 9 and 10 30 am and the afternoon clinic between 2 and 3 pm were invited to participate in the study. Patients were excluded if they were unable to understand the tests because of language difficulties or, for ease of statistical analysis, if they were unable to read, with one or both eyes, any of the letters presented on the charts. The exclusion of patients for whom visual acuities could not be recorded was necessary to allow more powerful parametric analyses to be carried out on the data.
A total of 132 patients were included in the study comprising 75 females and 57 males aged between 18 and 83 years (median 43 years). There were 81 patients with panuveitis, 34 with anterior uveitis, 13 with intermediate uveitis, and four with posterior uveitis classified according to the International Uveitis Study Group classification.17 The associated diseases/syndromes are shown in Table 1. Unilateral involvement was found in 50 patients and bilateral in 82, giving a total of 214 affected eyes; seven eyes were aphakic and 48 eyes pseudophakic. The treatment the patients were receiving is documented in Table 2, and the causes of visual loss are documented in Table 3.
Table 1.
Associated diseases/syndromes in 132 uveitis patients
| Associated syndromes | No of patients |
| Fuchs’ heterochromic cyclitis | 19 |
| Sarcoidosis | 13 |
| Behçet’s syndrome | 10 |
| Herpesvirus (herpes simplex type 1, varicella zoster) | 9 |
| Sclerouveitis | 8 |
| HLA-B27 | 7 |
| Vogt-Koyanagi-Harada syndrome | 3 |
| Demyelination | 2 |
| Toxoplasmosis | 2 |
| Systemic lupus erythematosus | 1 |
Table 2.
Causes of visual loss in 214 eyes of patients with uveitis
| Cause | No of eyes |
| Macular* | 63 |
| Vitritis | 38 |
| Glaucoma | 31 |
| Cataract | 28 |
| Refractive | 19 |
| Posterior capsular opacity | 18 |
| Corneal scarring | 5 |
| Deposits on intraocular lens | 3 |
| Amblyopia | 1 |
| Eyes with no sequelae | 42 |
*Includes cystoid macular oedema, macular ischaemia, epiretinal membrane, cellophane maculopathy, macular scar, macular hole.
Some eyes had more than one cause for visual loss.
Table 3.
ype of treatment in 132 patients with uveitis
| Treatment | No (%) |
| Systemic immunosuppression* | 4 (3) |
| Topical corticosteroid | 54 (41) |
| Systemic immunosuppression* and topical corticosteroid | 39 (30) |
| None | 35 (26) |
*Includes oral corticosteroid, cyclosporin A, azathioprine, methotrexate.
West Birmingham local research ethics committee approval was granted, and written consent obtained from each patient. There were no refusals to participate and an information leaflet was given to all patients. The study followed the tenets of the Declaration of Helsinki. Before commencing any tests, a synopsis of the study was explained to each patient by an ophthalmologist. Ophthalmological examination took place after the tests of visual function and quality of life measures were performed.
All patients were tested using their distance refractive correction if necessary. If the current treatments of the uveitis required dilatation of one or both pupils, then the tests were carried out under these conditions. The effects of pupil size, accommodation, and illumination on retinal image quality are well recognised,18–20 but we were assessing the patient under “real life” conditions.
Tests of visual function
Photopic lighting conditions were provided by two fluorescent strip lamps that were used to illuminate the test charts. All tests were carried out in the same room and in the same order by one examiner (AMG). The order of the tests of visual function was as follows:
Monocular high contrast visual acuity (VA worse eye/ better eye)
Binocular high contrast visual acuity (HCVA binocular)
Binocular low contrast visual acuity (LCVA binocular)
Contrast sensitivity (CS)
Vision Specific Quality of Life (VQOL) questionnaire.
High and low contrast visual acuity
High contrast (monocular and binocular) and low contrast (binocular) distance visual acuity was measured with an externally illuminated Bailey-Lovie chart21 positioned at a distance of 3 metres from the observer. The test distance was altered to 1 metre if a patient had very low vision. A Minolta spot photometer was used to ensure that chart luminance fell within the recommended range. To avoid familiarisation with the letters on the chart, two versions of the Bailey-Lovie charts were used.
Contrast sensitivity
Contrast sensitivity was measured using a Pelli-Robson chart.22 The chart was viewed binocularly, at a distance of 1 metre from the observer. The measurement procedure has been described previously.22,23 Patients were encouraged to guess if they could not see the letters, so the test was truly forced choice.24
Vision Specific Quality of Life Questionnaire
Vision related quality of life (perceived visual ability) was assessed using the core module (VCM1) of the Vision Specific Quality of Life (VQOL) questionnaire.16 The VCMI consists of 10 items that ask the patient general questions about the global quality of their vision and any concerns, anxieties, or problems they experience with regard to their visual impairment. Issues included embarrassment, anger, depression, loneliness, fear of deterioration in vision, safety in the home, safety outside the home, coping with everyday life, inability to do preferred activities, and life interference. The patients completed the VQOL questionnaire unless their eyesight was too poor to enable them to read it. If this was the case, the examiner administered the questionnaire. All patients were given the same instructions and the past month was used as the time frame for the questions. Each question had equal weighting; 0 indicating no problem and 5 indicating extreme problems. The final score was recorded as the arithmetic mean.
Statistical analysis
Pearson’s correlation coefficients (r) between the quality of life score and each visual function tested were calculated and a hypothesis test conducted to assess significance. The coefficient of determination (r2) was used to determine the percentage of the variance of quality of life score, which can be attributed to its linear regression on each visual variable. Individual correlations can be misleading because of intercorrelations between the variables. Hence, a stepwise multiple regression was performed by the forward method25 to identify which variables influenced quality of life score and their order of importance. We do not believe the data depart that much from normality to invalidate the tests used.
RESULTS
The range of scores obtained for each visual function test is shown in Table 4. VQOL score and HCVA (better eye) exhibit the highest and lowest variabilities respectively. In addition, HCVA (worse eye) exhibited a higher variability than HCVA (better eye).
Table 4.
Mean, standard deviation and range of test results taken from 132 patients with uveitis
| Variables | Mean (SD) | Range |
| HCVA (worse eye) | 0.37 (0.34) | −0.20 to1.38 |
| HCVA (better eye) | 0.11 (0.20) | −0.20 to 0.80 |
| HVCA (binocular) | 0.11 (0.23) | −0.20 to 0.90 |
| LCVA (binocular) | 0.36 (0.31) | −0.12 to1.20 |
| CS (binocular) | 1.62 (0.25) | 0.95 to 2.05 |
| VQOL | 1.7 (1.00) | 0.00 to 4.80 |
HCVA = high contrast visual acuity; LCVA = low contrast visual acuity; CS = contrast sensitivity; VQOL = Vision Quality Of Life.
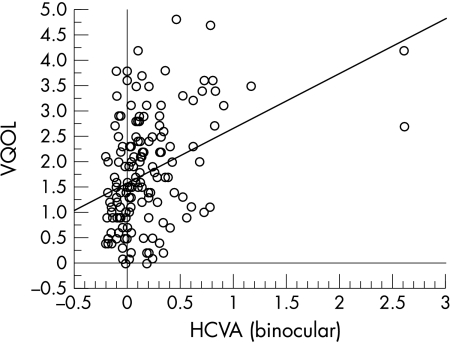
A linear regression plot of HCVA (binocular) against VQOL score is shown in Figure 1. The data show a wide scatter but the regression of HCVA (binocular) on VQOL is statistically significant (r = 0.28, p = 0.0011), suggesting that VQOL declined with reduced performance for HCVA (binocular); the regression accounting for 8% of the variance in VQOL. The correlation coefficients for the data as a whole are shown in Table 5. VQOL also declined with reduced performance for HCVA (worse eye) (r = 0.21, p = 0.015); HCVA (better eye) (r = 0.28, p = 0.0012), and LCVA (binocular) (r = 0.024, p = 0.0065). In addition, since low CS scores represent poor test performance, CS was the only visual function to exhibit an inverse relation with VQOL (r = 0.24, p = 0.005).
Figure 1.
Scatter plot showing relation between Vision Quality of Life (VQOL) and binocular high contrast visual acuity (BHVA).
Table 5.
Correlation coefficients, coefficients of determination, and probability values determined for correlations between various visual functions and quality of life scores
| VQOL | Correlation coefficient (r) | Coefficient of determination (r2) | Probability (P) |
| HCVA (worse eye) | 0.21 | 0.044 | 0.015 |
| HCVA (better eye) | 0.28 | 0.078 | 0.0012 |
| HCVA (binocular) | 0.28 | 0.080 | 0.0011 |
| LCVA (binocular) | 0.24 | 0.056 | 0.0065 |
| CS (binocular) | 0.24 | 0.059 | 0.005 |
HCVA = high contrast visual acuity; LCVA = low contrast visual acuity; CS = contrast sensitivity; VQOL = Vision Quality Of Life.
The results of the stepwise multiple regression analysis by the forward method are shown in Table 6. Only two X variables, HCVA (binocular) and age were selected as significantly related to VQOL; the two variables accounting for 15% of the variance in VQOL. In addition, extrapolating from the regression formula (VQOL = 2.34 + 1.57VA (binocular) − 0.02age) suggests that quality of life is particularly poor in younger patients with reduced binocular visual acuity.
Table 6.
Stepwise multiple regression analysis by the forward method of quality of life score (dependent variable Y) in relation to vision test scores and age (independent variables X1 to X6) in 132 patients with uveitis
| X selected | R | R2 | F | SSEx |
| HCVA binocular | 0.28 | 0.08 | 11.24* | 8% |
| Age | 0.39 | 0.15 | 11.98** | 7% |
Independent variables: high contrast visual acuity best eye (X1), high contrast visual acuity worst eye (X2), binocular high contrast visual acuity (HCVA binocular, X3), binocular low contrast visual acuity (X4), binocular contrast sensitivity (X5), and age (X6).
R = multiple regression coefficient, F = variance ratio, SSEx = percentage of the variance in Y associated with or explained by each X variable; *p<0.01, **p<0.001.
DISCUSSION
Uveitis can be a distressing and visually disabling disease. This study was designed to investigate the correlation between tests of visual function and perceived visual ability recorded with a quality of life questionnaire. We hoped to identify how uveitis affected the patient in the “real world” setting.
Regression analysis showed that all of the tests of visual function exhibited a statistically significant correlation with VQOL. Nevertheless, stepwise regression analysis revealed that HCVA (binocular) exerted the greatest influence on VQOL. Age was also statistically significantly associated with quality of life, with young people being more affected. This might be because visual deterioration in youth has the greatest impact on working life.
The correlation between perceived visual ability and visual function will, to some extent, be specific to the cause of reduced vision and the type of visual function affected by the disease. Previous studies, investigating the relation between visual function and quality of life, have also reported that HCVA had the highest correlation.26,27 Other studies, however, disagree. A study on patients with central field loss showed that LCVA was the best predictor of perceived visual ability.28 Binocular measurements of CS were most highly correlated with perceived visual disability in cataract patients.29
Perceived visual ability is not solely dependent on visual variables alone. Emotional or psychological factors also contribute to how well a patient believes he/she can see.30 Support for this comes from the observation that none of the visual functions tested in this study could account for more than 8% of the variance in VQOL.
A study on the psychological aspects of visual impairment,31 stated that a subjective perception of visual impairment is much more meaningful than objective measurements of vision. People create their own perceptions and often see the same situation very differently. Deterioration in the self reported quality of life of patients can be a result of anxiety that may occur prior to the stage where real difficulties are experienced.
A recent study32 showed that uveitis was associated with markedly reduced visual functioning using the NEI VFQ-25 questionnaire. General health status (using a SF-36 questionnaire) was also significantly lower in uveitis patients than in the general population.
The predominant cause of visual loss in our uveitis patients was macular pathology, particularly cystoid macular oedema, which is in agreement with other studies.1,6
It is clear from this study that uveitis has devastating effects on visual acuity and that this visual impairment interferes with the perceived visual ability of these patients, particularly in the younger patient. As most individuals with uveitis are of working age, employment, family, and social pressures may intensify the stress caused by visual impairment.
Adequate support from family, friends, clinicians and special organisations is essential in managing visual impairment. Some uveitis patients who suffer severe visual loss in one or both eyes are eligible for blind or partial sight registration Others, however, do not meet the criteria necessary for registration and yet these individuals may have a very poor perceived visual ability. Therefore, it is imperative that clinicians thoroughly examine the effects of uveitis on both visual functions and perceived visual ability when considering the management, treatment, and support of this group of patients.
Acknowledgments
This study was supported by a seeding grant from City Hospital NHS, Birmingham. We are grateful to Mr NA Frost for his assistance with the Vision Specific Quality of Life (VQOL) questionnaire.
REFERENCES
- 1.Rothova A, Suttorp-van Schulten MSA, Treffers WF, et al. Causes and frequency of blindness in patients with intraocular inflammatory disease.Br J Ophthalmol 1996;80:332–6.8703885 [Google Scholar]
- 2.Vadot E, Barth E, Billet P. Epidemiology of uveitis—preliminary results of a prospective study in Savoy. In: Saari KM, ed. Uveitis update. Amsterdam: Elsevier; 1984:13–16.
- 3.Department of Health and Social Security. Causes of Blindness and Partial Sight among adults in 1976/77, and 1980/81. London: HMSO, 1988.
- 4.Nussenblatt RB. The natural history of uveitis.Int Ophthalmol 1990;14:303–8. [DOI] [PubMed] [Google Scholar]
- 5.Research to Prevent Blindness. Annual report 1993. 598 Madison Avenue, New York, 1993:4.
- 6.Murray PI, Stavrou P, Marr JE, et al. Pattern of visual loss in patients with uveitis.Invest Ophthalmol Vis Sci 1998;39:S607 (abstract no 2812). [Google Scholar]
- 7.Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of “real world” targets.Br J Ophthalmol 1987;71:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owsley C, Ball K, Sloane ME, et al. Visual/cognitive correlates of vehicle accidents in older drivers.Psychol Aging 1991;6:403–15. [DOI] [PubMed] [Google Scholar]
- 9.Marron JA,Bailey IL. Visual factors and orientation-mobility performance.Am J Optom Physiol Opt 1982;5:413–26. [DOI] [PubMed] [Google Scholar]
- 10.Wood JM, Troutbeck R. The effect of visual impairment on driving.Hum Factors 1994;36:476–87. [DOI] [PubMed] [Google Scholar]
- 11.Elliott DB, Whitaker D. How useful are contrast sensitivity charts in optometric practice.Optom Vis Sci 1992;69:378–85. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg EP, Tielsch JM, Schein DO, et al. The VF-14 An index of functional impairment in patients with cataract.Arch Ophthalmol 1994;112:630–8. [DOI] [PubMed] [Google Scholar]
- 13.Cassard SD, Patrick DL, Damiano AM. Reproducibility and responsiveness of the VF-14.Arch Ophthalmol 1995;113:1508–13. [DOI] [PubMed] [Google Scholar]
- 14.Mangione CM, Phillips RS, Seddon JM, et al. Development of the “Activities of Daily Vision Scale”: a measure of visual functional status.Med Care 1992;30:1111–26. [DOI] [PubMed] [Google Scholar]
- 15.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire.Arch Ophthalmol 2001;119:1050–8. [DOI] [PubMed] [Google Scholar]
- 16.Frost NA, Sparrow JM, Durant JS, et al. Development of a questionnaire for measurement of vision-related quality of life.Ophthalmic Epidemiol 1998;5:185–210. [DOI] [PubMed] [Google Scholar]
- 17.Bloch-Michel E, Nussenblatt RB. International uveitis study group recommendations for the evaluation of intraocular inflammatory disease.Am J Ophthalmol 1987;103:234–6. [DOI] [PubMed] [Google Scholar]
- 18.Walsh G. The effect of mydriasis on the pupillary centration of the human eye.Ophthal Physiol Opt 1987;8:178–82. [DOI] [PubMed] [Google Scholar]
- 19.Walsh G, Charman WN. the effect of pupil centration and diameter on ocular performance.Vis Res 1988;28:659–65. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MA, Simonet P. Change of pupil centration with change of illumination and pupil size.Optom Vis Sci 1992;69:129–36. [DOI] [PubMed] [Google Scholar]
- 21.Bailey IL, Lovie JE. New design principles for visual acuity letter charts.Am J Optom Physiol Opt 1976;53:740–5. [DOI] [PubMed] [Google Scholar]
- 22.Pelli DG, Robson JG, Wilkins AJ. The design of a new chart for measuring contrast sensitivity.Clin Vis Sci 1988;2:187–99. [Google Scholar]
- 23.Elliott DB, Sanderson K, Conkey A. The reliability of the Pelli-Robson contrast sensitivity chart.Ophthal Physiol Opt 1990;10:21–4 [PubMed] [Google Scholar]
- 24.Vaegan, Halliday BL. A forced-choice test improves clinical contrast sensitivity testing.Br J Ophthalmol 1982;66:477–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snedicor GW, Cochran WG. Statistical Methods. Iowa State University Press 1980:
- 26.Wu AW, Coleson LC, Holbrook J, et al. Measuring visual function and quality of life in patients with cytomegalovirus retinitis.Arch Ophthalmol 1996;114:841–7. [DOI] [PubMed] [Google Scholar]
- 27.Szlyk JP, Fishman GA, Alexander KR, et al. Relationship between difficulty in performing daily activities and clinical measures of visual function in patients with retinitis pigmentosa.Arch Ophthalmol 1997;115:53–9. [DOI] [PubMed] [Google Scholar]
- 28.Hazel CA, Petre KA, Armstrong RA, et al. Visual function and subjective quality of life compared in subjects with acquired macular disease.Invest Ophthalmol Vis Sci 2000;41:1309–15. [PubMed] [Google Scholar]
- 29.Elliott DB, Hurst MA, Weatherill J. Comparing clinical tests of visual function in cataract with the patients perceived visual disability.Eye 1990;4:712–7. [DOI] [PubMed] [Google Scholar]
- 30.Connor GB, Muldoon JF. A statement of the needs of blind and visually impaired individuals.New Outlook for the Blind 1973;67:352–62. [Google Scholar]
- 31.Baus S. Psychological aspects of visual impairment.J Vis Impair Blindness 1999;17:41–4. [Google Scholar]
- 32.Schiffman RM, Jacobsen G, Whitcup SM. Visual functioning and general health status in patients with uveitis.Arch Ophthalmol 2001;119:841–9. [DOI] [PubMed] [Google Scholar]