Abstract
Aims: To examine whether the early postoperative morphology at the site of sclerectomy, as visualised by ultrasound biomicroscopy (UBM), is an indicator of the mechanisms that lower intraocular pressure (IOP) and/or predictors of the long term outcome of viscocanalostomy.
Methods: 15 eyes of 14 patients with medically uncontrolled open angle glaucoma and no history of surgery underwent viscocanalostomy according to Stegmann’s technique. Ultrasound biomicroscopy was performed during the first month after surgery. The following parameters were assessed: dimensions of the intrascleral “lake,” presence of a filtering bleb, presence of a subconjunctival cavity or a suprachoroidal hypoechoic area, and the thickness of the residual trabeculocorneal membrane. A complete ophthalmological examination was performed the day before and the day after surgery. Follow up visits were scheduled 1 week, 4 weeks, 6 months, and 12 months after surgery.
Results: At 1 year successful control of IOP (<20 mm Hg) was achieved without further manipulation or medication in six of 15 eyes. The size of the intrascleral “lake” (average 0.62 mm3) did not correlate with later IOP; however, a visible route under the scleral flap and accidental perforation of the trabeculocorneal membrane were associated with long term lowering of IOP. Normal thickness of the trabeculocorneal membrane (0.10–0.15 mm) was indicative of IOP control with and without medication. When UBM showed an early collapse of the intrascleral cavity, control of IOP was not achieved. Other UBM findings did not predict long term function.
Conclusion: In accordance with previous studies, the authors found that UBM examination is a useful method to evaluate outflow mechanisms after glaucoma surgery. This study shows that UBM imaging of external filtration during the early postoperative period can be used to predict the success of viscocanalostomy. However, to establish conclusively what parameters of UBM predict successful viscocanalostomy a larger number of patients must be studied.
Keywords: high frequency ultrasound, ultrasound biomicroscopy, viscocanalostomy, non-penetrating glaucoma surgery
Several investigators have shown renewed interest in surgical reduction of intraocular pressure (IOP) by non-perforating glaucoma surgery. Non-perforating glaucoma surgery avoids opening the anterior chamber and decompressing the eye, thus circumventing many serious complications associated with standard trabeculectomy.1 In open angle glaucoma, the endothelium of Schlemm`s canal and the immediately adjacent trabecular meshwork show increased resistance to aqueous outflow,2 resulting in increased IOP. Recently, a new technique of non-penetrating glaucoma surgery, viscocanalostomy, has been described; it results in better outflow in open angle glaucoma.3,4 In this procedure Schlemm’s canal is unroofed and Descemet’s membrane is separated 1–2 mm from the corneoscleral junction, resulting in a thinner but intact window to the anterior chamber, through which aqueous humour diffuses into a subscleral lake created by the removal of an inner scleral flap. Filtration is improved when the diameter of Schlemm’s canal is enlarged by the injection of a high viscosity viscoelastic material into the opened ostia of the canal.
The nature of the outflow pathways that lead to the lowering of IOP in viscocanalostomy surgery is controversial.5,6 Several mechanisms may be involved: these include permanent subconjunctival filtration (as in trabeculectomy), aqueous flow into the canalicular system that reaches the venous circulation or the uveoscleral space, with or without an intrascleral “lake,” and drainage from Schlemm’s canal to capillaries and veins within the intrascleral canals and subconjunctival tissue.
Morphological studies have shown varying dissection depths of the deep scleral flap that often leads to an unroofing of Schlemm’s canal.7,8
Postsurgical examination with the high resolution ultrasound biomicroscope (UBM), developed by Pavlin and Foster,9 allows imaging of the trabeculo-descemetic membrane, the intrascleral hypoechoic cavity and subconjunctival filtration (clinically not visible during slit lamp examination) with a resolution of 50 μm.10–12 UBM examination can also detect the presence of small amount of fluid, such as subchoroidal effusion, between layers of the eye.13
The aim of the present study was to analyse the aqueous drainage pathways under the scleral flap and to examine the presence and dimensions of the subconjunctival and suprachoroidal space in eyes that underwent viscocanalostomy. In this study UBM findings were used to evaluate potential predictive parameters with reference to long term success or failure of viscocanalostomy.
PATIENTS AND METHODS
In this retrospective study we enrolled 15 eyes of 14 patients with uncontrolled open angle glaucoma. The patients had no history of glaucoma surgery or laser treatment and all had had maximal medical therapy without success. Included were patients with pseudoexfoliation glaucoma (n = 7), pigment dispersion glaucoma (n = 4) and primary open angle glaucoma (n = 4) (Table 1). Exclusion criteria were secondary or dysgenetic glaucoma, narrow angle glaucoma, a legally blind fellow eye, or corneal abnormalities that prevented reliable applanation tonometry.
Table 1.
Patient data
| UBM findings | ||||||||||||||||
| Patient No | M/F | Age | Diag | Operation date | Lake (mm3) | FB | Chor | Subcon | Route | TMW | Clin | Success (time lapse for failure (days)) | Further manip | IOPmax (mm Hg) | IOPpre (mm Hg) | IOPpost (mm Hg) |
| 1 | F | 63 | PEX | 28.06.99 | 0.16 | + | + | + | − | ++++ | yes | 34 | 22 | 16 | ||
| 2 | F | 63 | PEX | 25.06.99 | 1.44 | ++ | − | + | ++ | 0.15 perf | yes | 30 | 22 | 18 | ||
| 3 | F | 67 | PEX | 06.07.99 | 0.25 | + | + | + | (+) | 0.15 b | FB | no (268) | med | 24 | 24 | 20 |
| 4 | F | 64 | PEX | 05.08.99 | 0.60 | ++ | − | − | − | Collaps | no (1) | surg | 45 | 40 | 14 | |
| 5 | M | 54 | PEX | 09.07.99 | 0.22 | (+) | − | + | − | 0.15 | FB | no (186) | med | 29 | 24 | 16 |
| 6 | M | 34 | PIG | 16.07.99 | >0.1 | + | − | + | − | Collaps | no (71) | surg | 45 | 30 | 14 | |
| 7 | F | 58 | POAG | 11.05.99 | >0.1 | + | + | − | − | Collaps | FB | no (5) | surg | 30 | 22 | 38 |
| 8 | M | 64 | POAG | 05.07.99 | 0.17 | (+) | − | + | (+) | ++++ | no (6) | surg | 32 | 23 | 16 | |
| 9 | F | 56 | PIG | 17.08.99 | 0.76 | ++ | + | + | − | 0.1 | FB leakage | yes | 24 | 23 | 9 | |
| 10 | F | 63 | POAG | 08.09.99 | 2.54 | +++ | − | + | (+) | 0.1 perf | FB | yes | 26 | 22 | 16 | |
| 11 | F | 66 | PEX | 17.09.99 | 0.22 | + | ++ | + | + | 0.15 | FB | yes | 50 | 26 | 16 | |
| 12 | M | 52 | PIG | 22.09.99 | 0.26 | ++ | − | − | − | Collaps | no (16) | surg | 38 | 25 | 12 | |
| 13 | M | 55 | PEX | 11.10.99 | 0.75 | − | + | − | − | 0.05 b | FB | no (41) | med | 37 | 28 | 24 |
| 14 | F | 62 | POAG | 13.12.99 | 0.10 | ++ | − | − | − | 0.1 | FB | yes | 34 | 22 | 13 | |
| 15 | M | 44 | PIG | 03.02.00 | 1.82 | ++ | − | − | − | 0.1 | FB | no (333) | (med) | 55 | 26 | 30 |
Diag = ocular diagnosis, lake = intrascleral cavity, FB = filtering bleb, chor = suprachoroidal hyporeflexive zone, subcon = subconjunctival hypoechoic cavity, route = visible aqueous route under scleral flap, TMW = trabecular meshwork and adjacent tissue, clin = clinically visible filtering bleb (FB), further manip = further manipulation, med = glaucoma medication, surg = further surgery, IOPmax = maximum value of IOP, IOPpre = IOP before surgery, IOPpost = IOP after surgery at final visit, rows in bold type = successful viscocanalostomy with IOP<20 mm Hg after 1 year of follow up.
A complete ophthalmological examination was performed the day before and the day after surgery. The assessment included IOP measurement, visual acuity, gonioscopy, and anterior and posterior slit lamp examination. Follow up visits were scheduled at 1 week, 4 weeks, 6 months, and 12 months after surgery. Written informed consent was obtained from all subjects.
Patients were eight women and six men aged 34–67 years (mean 57.6 years). Between June 1999 and March 2000 these patients underwent viscocanalostomy according to the technique of Stegmann et al3 at the centre of ophthalmology, Cologne, Germany. Two experienced surgeons (WK and PCJ) performed all surgical procedures. No antiproliferative agents were used for the first surgical procedure.
UBM examination
An ultrasound biomicroscope (Humphrey Inc, Zeiss Group, Jena) model 840 was used in this study. With the patients in a supine position and with the aid of a lid speculum and an examination gel of low viscosity the surgical area was scanned with the 50 MHZ probe. UBM was performed during the first month after surgery. The following parameters were analysed: the maximal height, the maximal radial and transverse dimensions of the intrascleral lake, the presence or absence of a filtering bleb or subconjunctival cavity, the presence or absence of a suprachoroidal hypoechoic area, and the thickness of the residual trabeculocorneal membrane. To avoid bias all examinations were performed once each by two experienced ophthalmologists.14
Viscocanalostomy procedure
A fornix based conjunctival flap was prepared, Tenon’s capsule was opened, and both were retracted to expose the sclera. Haemostasis was maintained by irrigation with balanced salt solution to avoid damage and scarring of the anatomical structures. A 5 × 5 mm triangular superficial flap was prepared and a second 4 × 4 mm deeper scleral flap deroofed Schlemm’s canal. This inner flap was pulled upward, and the floor of the canal and Descemet’s membrane were depressed with the tip of a cellulose sponge; the membrane was then separated from the cornea for a distance of about 1–2 mm. Afterwards the inner scleral flap was excised. After cannulation and correct positioning of the cannula using gonioscopy, the proximal ends of Schlemm’s canal and the intrascleral space were filled with sodium hyaluronate (Healon GV) according to Stegmann’s technique. Afterwards, the inner flap was cut off and the superficial flap was closed tightly with 10-0 Nylon sutures. To prevent early scarring, Healon GV was injected underneath the flap. The conjunctiva was closed with 7-0 Vicryl sutures adjacent to the limbus. Immediately after surgery 40 mg of gentamicin (1 cm3, 1:4 dilution) and 2 mg betamethasone were injected subconjunctivally. During postoperative recovery, each subject was administered a preservative-free local steroid five times a day and a combination of steroid and antibiotic ointment at night.
Surgical success was defined as IOP less than 20 mm Hg without the need of for topical medication or additional surgery.
RESULTS
The mean preoperative IOP was 25.26 mm Hg (range 22–40 mm Hg). After 1 year, six of 15 patients reached and maintained an IOP of ≤20 mm Hg without further surgery or medication. The mean IOP after 1 week was 16.1 (n=13), after 4 weeks was 14.6 mm Hg (n=10), after 6 months was 16.6 mm Hg (n=15), and after 12 months (including those patients who required drug therapy and those requiring additional surgery) the mean IOP was 18.2 mm Hg (n=15) (Tables 1 and 2).
Table 2.
IOP at the scheduled follow up visits
| Patient No | Maximal (mm Hg) | Before (mm Hg) | 1 week (mm Hg) | 4 weeks (mm Hg) | 6 months (mm Hg) | 12 months (mm Hg) |
| 1 | 34 | 22 | 14 | 16 | 16 | |
| 2 | 30 | 22 | 10 | 18 | 18 | |
| 3 | 24 | 24 | 16 | 18 | 20 | 20 |
| 4 | 45 | 40 | 30 | 20 | 14 | |
| 5 | 29 | 24 | 14 | 15 | 17 | 16 |
| 6 | 45 | 30 | 8 | 10 | 14 | |
| 7 | 30 | 22 | 31 | 19 | 17 | 38 |
| 8 | 32 | 23 | 23 | 16 | 16 | |
| 9 | 24 | 23 | 12 | 9 | 9 | |
| 10 | 26 | 22 | 6 | 13 | 16 | 17 |
| 11 | 50 | 26 | 12 | 13 | 13 | 16 |
| 12 | 38 | 25 | 17 | 15 | 32 | 12 |
| 13 | 37 | 28 | 19 | 17 | 12 | 24 |
| 14 | 34 | 22 | 10 | 10 | 16 | 13 |
| 15 | 55 | 26 | 14 | 18 | 30 |
maximal = maximum value of IOP; rows in bold type = successful viscocanalostomy with IOP 20 mm Hg after 1 year of follow up.
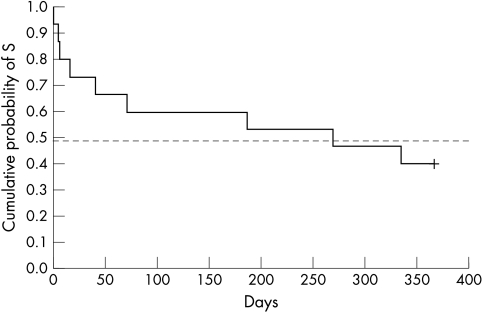
No serious complications or long lasting side effects were noted in the operated eyes. Intraoperative complications were perforation of the trabeculocorneal membrane in two eyes. Postoperative complications included a leaking bleb in one eye, which closed spontaneously, and transient hyphaema of 1 mm and 2 mm in two eyes. Complications due to overfiltration, such as flat anterior chamber, prolonged ocular hypotony, and choroidal detachment were not observed. Significant cataract formation with a drop in visual acuity also was not observed. IOP spikes occurred in nine eyes; five of these eyes required additional surgery, three required drug therapy, and the patient in whom the spike was observed at the last follow up visit was instructed to use drug therapy. In two patients who had laser suturelysis, IOP control could not be achieved. The Kaplan-Meier curve shows the cumulative probability of success until IOP exceeded 20 mm Hg (Fig 1).
Figure 1.
Kaplan-Meier curve for all subjects (n=15). Time lapse until IOP exceeds 20 mmHg. Cumulative probability of survival.
UBM was performed on average 12 days after viscocanalostomy and showed seven different morphological characteristics.
Intrascleral lake
A visible intrascleral hypoechoic zone was observed in 13 eyes (86.6%). All 13 eyes achieved an IOP of ≤20 mm Hg; of these, six required no additional treatment, four drug therapy, and three additional surgery.
A large intrascleral lake (>0.50 mm3) was observed in six eyes (40%). All six eyes achieved an IOP of ≤20 mm Hg; of these, three required no additional treatment, two drug therapy, and one additional surgery.
The dimension of the intrascleral lake (average 0.62 mm3) did not correlate with changes in IOP (Figs 2 and 3).
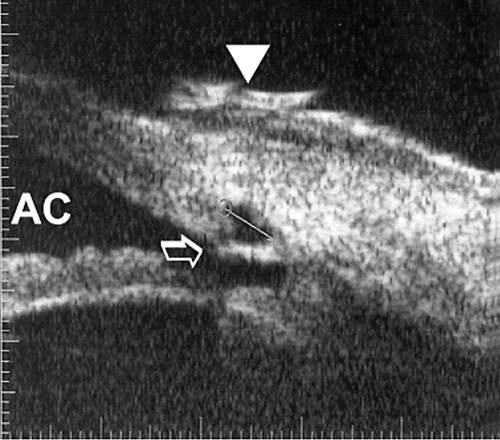
Figure 2.
UBM (radial section) 28 days after viscocanalostomy. A small perforation of residual trabecular meshwork (arrow), the small intrascleral “lake” (volume 0.36 mm3), a small low reflective filtering bleb (arrowhead), and a convex iris configuration were visualised by UBM. AC = anterior chamber.
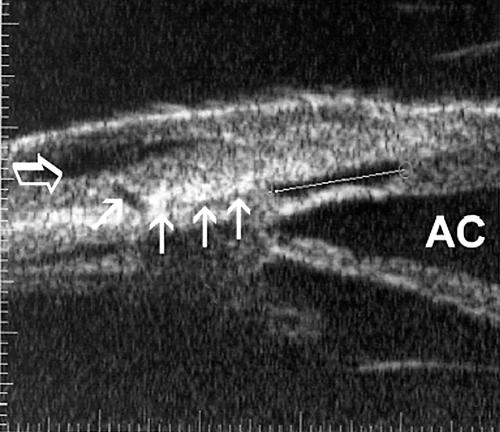
Figure 3.
UBM (radial section) 8 days after viscocanalostomy. An intact trabeculo-descemetic membrane (0.15 mm), an intrascleral “lake” (volume 0.63 mm3 with the calipers measuring maximum radial dimension), a small “route” under the scleral flap to a subconjunctival cavity (small arrows) and a hyporeflexive subconjunctival cavity (translucent arrow) could be visualised. AC = anterior chamber.
Low reflective filtering bleb
A filtering bleb was observed in 14 eyes (93.3%). A filtering bleb was not observed in only one eye, which required drug therapy.
A large filtering bleb was observed in four of six eyes that achieved an IOP of ≤20 mm Hg; one of the eyes required drug therapy and two required additional surgery.
Subchoroidal hypoechoic area
A subchoroidal space was observed in six eyes (40%): all six eyes achieved an IOP of ≤20 mm Hg; of these, three did not require additional treatment, one required drug therapy, and two additional surgery.
Subconjunctival hyporeflexive cavity
A hyporeflexive subconjunctival cavity was imaged in nine eyes (60%). All nine eyes achieved an IOP of ≤20 mm Hg; of these, five required no additional treatment, two drug therapy and two additional surgery (Fig 3).
Visibility of the route under the scleral flap
Separation of the scleral flap owing to a presumed fluid stream was observed in two of the eyes that achieved an IOP of ≤20 mm Hg without any additional treatment. A very small route (diameter <0.05 mm) occurred in one eye in each group (Fig 3).
Thickness of remaining trabecular meshwork
UBM examination showed perforation of the trabeculocorneal membrane (Fig 2) in two eyes that achieved an IOP of ≤20 mm Hg without additional treatment. A postoperative thickness of 0.10–0.15 mm was measured in five of six eyes that achieved an IOP of ≤20 mm Hg without additional treatment and in the four eyes that required drug therapy (66.6%). A thickened trabeculocorneal membrane (>0.3 mm) was observed in one eye that required additional surgery (13.3%).
Collapse of trabecular meshwork
An early collapse of the superficial scleral flap with residual trabecular meshwork was observed in four of five eyes only in the group that required additional surgery (26.6%).
A clinically visible filtering bleb was observed in nine eyes (60%). All nine eyes achieved an IOP of ≤20 mm Hg; of these, four required no additional treatment, four required drug therapy, and one required additional surgery.
DISCUSSION
Non-penetrating surgery was first introduced in 1984 by Fjodorov et al15 and in 1989 by Koslov et al.16 Stegmann et al3 modified the procedure by injecting viscoelastic material into Schlemm’s canal. The Stegmann procedure, named viscocanalostomy, was thought to give better IOP control in black subjects than conventional filtration surgery.
The features of viscocanalostomy include preparation of a deep scleral lamella after a superficial one, and creating a “Descemet’s window” with anterior preparation of the deep lamella into the cornea just above Descemet’s membrane. Schlemm’s canal is deroofed, the deep lamella is dissected and the open ends of Schlemm’s canal are filled with viscoelastic material. Percolation of aqueous through Descemet’s window was supposed to be indicative of proper preparation.17 Early reports of favourable results3,4 were accompanied by discussions about the mechanisms by which such a procedure lowers IOP, particularly because of the extremely low permeability of Descemet’s membrane for water and because the sites of resistance to outflow (juxtacanalicular meshwork and the inner wall of Schlemm’s canal) are kept intact throughout the procedure.18 Consequently other outflow pathways must be involved.19
UBM permits visualisation of the postoperative area and measurement of the thickness of the trabeculocorneal membrane and the dimensions of the intrascleral lake.20 Despite an axial and lateral resolution capacity of 50 μm, imaging of the exact preparation level is not possible. In this study the volume of the intrascleral lake was determined by multiplying the maximum height by the limbus parallel and radial extension, resulting in a quadratic equation. This equation was chosen according to previous studies,21 although a more precise calculation would be that for lenticular objects.
Under physiological conditions, 85% of aqueous outflow is transtrabecular.22 Sclerectomy and viscocanalostomy lead to the reduction of outflow resistance via a number of mechanisms, which include23:
thinning of the trabecular meshwork
vaulting of residual trabecular meshwork vaults towards the intrascleral cavity leading to a widening of the cribriform interspace (similar to laser trabeculoplasty)
opening or widening of Schlemm’s canal inner wall and of the juxtatrabecular meshwork by injection of viscoelastic.
Other unconventional outflow mechanisms that may be improved by surgery include:
scleral thinning when a superficial scleral flap permits passage into the subconjunctival space
formation of an intrascleral lake, which leads to formation of aqueous veins
trans-scleral filtration into the supraciliary/suprachoroidal space.
Accidental lesions that can lead to higher aqueous outflow include:
opening of a cyclodialysis clefts (these may only be observed by UBM examination)24
localised choroidal effusion
opening of the anterior chamber.
In our studies the residual trabeculo-descemetic membrane was unstable if its thickness was ≤0.05 mm. The inward vaulting into the intrascleral cavity led to collapse of the lumen of the cavity and an increase in IOP. A trabeculo-descemetic membrane is stable when the thickness is 0.13 (SD 0.02) mm.21 A visible Schlemm’s canal following viscoelastic injection was never observed, even when UBM examination was performed on the second postoperative day.
The inability to view Schlemm’s canal and its degree of patency is a limitation of commercially available UBM technology. Without that information, one aspect of the mechanism (flow into a dilated Schlemm’s canal) cannot be addressed.
It would be expected that a large intrascleral lake would result in good trans-scleral and suprachoroidal filtration as well as increased contact with aqueous veins but, in fact, the size of the intrascleral lake did not correlate with IOP control.25,26
Small perforations that open a hole in the anterior chamber result in a successful viscocanalostomy; however, unpublished observations indicate that large perforations lead to adhesions of the peripheral iris or collapse of the intrascleral lake and an increase in IOP.
In this study UBM of non-penetrating sclerectomy with viscocanalostomy showed seven significant postoperative findings. Of these findings, a visible intrascleral lake, which was found in 13 of 15 eyes (including those in which the procedure failed), a suprachoroidal hypoechoic area visualised in the early postoperative period and a large intrascleral cavity were not prognostic of long term function. Earlier ultrasound biomicroscopic studies have shown that blebs of the L-type (low reflective) are associated with good IOP control in trabeculectomised eyes11,12 with adjunctive mitomycin C.27 In this study filtering blebs were found in all but one eye 1 month after surgery; however, even a large hyporeflexive filtering bleb was not indicative of good IOP control, and in fact two eyes needed further surgical intervention. A hyporeflexive subconjunctival cavity was often found in successful viscocanalostomies; however, two eyes with a hyporeflexive subconjunctival cavity needed additional surgery.
A UBM finding that was indicative of successful IOP control was an easy visible “route” under the scleral flap; however, if the diameter of the route was <0.05 mm, there was no guarantee for long term function. Earlier studies have shown that the thickness of the aqueous drainage route beneath the scleral flap influences the development of a filtering bleb, and both are indicative of good IOP control (follow up mean 9.9 months).11,12 During the 1 year follow up period, control of IOP, with or without the addition of drug therapy, was successful when the residual trabeculocorneal membrane measured between 0.10 mm and 0.15 mm and when a filtering bleb was observed clinically. The clinical finding of a filtering bleb was not necessarily observable from UBM imaging, and in cases with large hyporeflexive blebs shown on UBM, the clinical examination did not indicate the presence of a filtering bleb despite evaluation by four experienced ophthalmologists (two for clinical examination and two for UBM examination).
Early studies of viscocanalostomy claimed that this procedure was not as efficient for controlling IOP in white glaucoma patients as standard filtration surgery and was therefore indicated for its safety more than for its efficacy. Comparison of trabeculectomy and viscocanalostomy showed that during a 6 month follow up, IOP was successfully controlled in five of 10 eyes in the trabeculectomy group and in none of the eyes in the viscocanalostomy group.28 However, Carassa et al4 reported a success rate of 86.2% after 12 months in eyes that underwent viscocanalostomy. In summary, these studies are difficult to compare because of differences in the criteria of successful control of IOP and varying follow up periods.
Numerous surgical modifications, such as laser goniopuncture, trabecular stripping, trabecular microperforation, and various implants into the scleral space have been introduced to decrease IOP and reduce outflow resistance in trabecular meshwork and/or canaliculation of aqueous flow into the subconjunctival area. Johnson and Johnson2 identified microruptures in the trabecular meshwork after non-penetrating surgery bypassing the resistance of the juxtacanalicular meshwork, explaining why many patients who have had successful non-penetrating operations developed filtering blebs. Drüsedau et al29 reported that none of the eyes in a series of 41 viscocanalostomies with microperforation required further pressure lowering surgery, whereas five of the “perfect” surgeries needed additional surgery. These results support our findings that perforation of the trabecular meshwork and an aqueous route under the scleral flap indicate successful IOP control similar to that obtained with standard filtration surgery.
As far as the safety of viscocanalostomy is concerned, there are certainly fewer complications, such as postoperative hypotensive complications, flat anterior chamber, and choroidal detachment; however, hyaluronate detachment of Descemet’s membrane can occur.30
UBM is a useful method for assessing the anatomical changes in eyes undergoing viscocanalostomy, which may allow an understanding of the outflow mechanisms. In spite of many variations observed, it may be stated that non-penetrating viscocanalostomy works best when ultrasound biomicroscopic observations are similar to those observed after filtration surgery. A study with later UBM is under way, in order to compare the findings with the early UBM appearance. However, a larger number of patients needs to be studied to define what parameters predict successful IOP control.
REFERENCES
- 1.Mermoud A, Schnyder CC, Sickenberg M, et al. Comparison of deep sclerectomy with collagen implant and trabeculectomy in open angle glaucoma.J Cataract Refract Surg 1999;25:323–31. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DH, Johnson M. How does nonpenetrating glaucoma surgery work? Aqueous outflow resistance and glaucoma surgery.J Glaucoma 2001;10:55–67. [DOI] [PubMed] [Google Scholar]
- 3.Stegmann RC, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black patients.J Cataract Refract Surg 1999;25:316–21. [DOI] [PubMed] [Google Scholar]
- 4.Carassa RG, Bettin P, Fiori M, et al. Viscocanalostomy: a pilot study.Eur J Ophthalmol 1998;8:57–61. [DOI] [PubMed] [Google Scholar]
- 5.Krieglstein GK. How new is new, and is it better? (Editorial).J Glaucoma 1999;8:6–11. [PubMed] [Google Scholar]
- 6.Sourdille P. Nonpenetrating glaucoma surgery: it’s worth the change (review).J Cataract Refract Surg 1999;25:298–300. [DOI] [PubMed] [Google Scholar]
- 7.Dietlein TS, Lüke C, Jacobi PC, et al. Variability of disssection depth in deep sclerectomy – morphological analysis of the deep scleral flap.Graefes Arch Clin Exp Ophthalmol 2000;238:405–9. [DOI] [PubMed] [Google Scholar]
- 8.Dietlein TS, Lüke C. Jacobi PC, et al. Does the dissection depth and thickness of the deep scleral flap influence the intraocular pressure after viscocanalostomy? A clinico-pathologic correlation.Klin Monatsbl Augenheilkd 2001;218:168–73. [DOI] [PubMed] [Google Scholar]
- 9.Pavlin CJ, Sherar MD, Foster FS. Subsurface ultrasound microscopic imaging of the intact eye.Ophthalmology 1990;97:244–50. [DOI] [PubMed] [Google Scholar]
- 10.Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes.Am J Ophthalmol 1992;113:381–9. [DOI] [PubMed] [Google Scholar]
- 11.Jinza K, Saika S, Kin K, et al. Relationship between formation of a filtering bleb and an intrascleral aqueous drainage route after trabeculectomy: evaluation using ultrasound biomicroscopy.Ophthalmic Res 2000;32:240–3. [DOI] [PubMed] [Google Scholar]
- 12.Avitabile T, Uva MG, Russo V, et al. Ultrasound biomicroscopic evaluation of filtering blebs.Klin Monatsbl Augenheilkd 1998;212:101–5. [DOI] [PubMed] [Google Scholar]
- 13.Pavlin CJ, Harasiewicz K, Sherar, MD, et al. Clinical use of ultrasound biomicroscopy.Ophthalmology 1991;98:287–95. [DOI] [PubMed] [Google Scholar]
- 14.Tello C, Liebmann J, Potash SD, et al. Measurement of ultrasound biomicroscopy images: intraobserver and interobserver reliability.Invest Ophthalmol Vis Sci 1994;35:3549–52. [PubMed] [Google Scholar]
- 15.Fjodorov SN, Ioffe DI, Ronkina TI. Deep sclerectomy: technique and mechanism of a new glaucomatous procedure.Glaucoma 1984;6:281–3. [Google Scholar]
- 16.Kozlov VI, Bagrov SN, Anisimova SY, et al. Non penetrating deep sclerectomy with collagen. IRTC Eye Microsurgery. Moscow: RSFSR Ministry of Public Health 1989;3:44–6.
- 17.Boyd BF. The role of non-penetrating filtering operations for open angle glaucoma.Highlights Ophthalmol 2000;4:29–31. [Google Scholar]
- 18.Grant WM. Experimental aqueous perfusion in enucleated human eyes.Arch Ophthalmol 1963;69:783–801. [DOI] [PubMed] [Google Scholar]
- 19.Kotliar KE, Mertz M, Lanzl M. Possible outflow mechanisms after deep sclerectomy.Ophthalmologe 2000;97(suppl 1):S15. [Google Scholar]
- 20.Chiou AGY, Mermoud A, Underdahl JP, et al. An ultrasound biomicroscopy study of eyes after deep sclerectomy with collagen implant.Ophthalmology 1998;105:746–50. [DOI] [PubMed] [Google Scholar]
- 21.Chiou AGY, Mermoud A, Hédiguer S-EA, et al. Ultrasound biomicroscopy of eyes undergoing deep sclerectomy with collagen implant.Br J Ophthalmol 1996;80:541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grehn F, Mackensen G. Die Glaukome. Kohlhammer-Verlag, Stuttgart, Berlin, Köln, 1993.
- 23.Schwenn O, Dick B, Pfeiffer N: Trabeculectomy, deep sclerectomy and viscocanalostomy.Ophthalomologe 1998;93:835–43. [DOI] [PubMed] [Google Scholar]
- 24.Klemm M, Bergmann U, Guthoff. Ultrasound biomicroscopic imaging for assessment of the suprachoroidal cleft after angle surgery.Klin Monatsbl Augenheilkd 1999;210:74–7. [Google Scholar]
- 25.Sannace C, Miserocchi E, Carassa RG, et al. Viscocanalostomy: an ultrasound biomicroscopic study.Invest Ophthalmol Vis Sci 2000;41:S578. [Google Scholar]
- 26.Hermel M, Arend O, Plange N, et al. Ultrasound biomicroscopic findings after non-penetrating sclerectomy with viscocanalostomy.Klin Monatsbl Augenheilkd 2000;217(suppl 7):2–3. [Google Scholar]
- 27.Yamamoto T, Sakuma T, Kitazawa Y. An ultrasound biomicroscopic study of filtering blebs after mitomycin C trabeculectomy.Ophthalmology 1995;102:1770–6. [DOI] [PubMed] [Google Scholar]
- 28.Jonescu-Cuypers C, Jacobi PC, Konen W, et al. Primary viscocanalostomy versus trabeculectomy in white patients with open-angle glaucoma: A randomized clinical trial.Ophthalmology 2001;108:254–8. [DOI] [PubMed] [Google Scholar]
- 29.Drüsedau MU, Von Wolff K, Bull H, et al. Viscocanalostomy for primary open-angle glaucoma: the Gross Pankow experience.J Cataract Refract Surg 2000;26:1367–73. [DOI] [PubMed] [Google Scholar]
- 30.Lüke C, Dietlein TS, Jacobi PC, et al. Intracorneal inclusion of high-molecular-weight sodium hyaluronate following detachment of Descemet’s membrane during viscocanalostomy.Cornea 2000;19:556–7. [DOI] [PubMed] [Google Scholar]