Abstract
Background/aims: T lymphocytes are present in increased numbers in the conjunctiva of patients with vernal keratoconjunctivitis (VKC) and their activation has a central role in the pathogenesis of the chronic allergic inflammatory reactions seen in VKC. The aims of this study were to examine the expression of three recently described potent T lymphocyte chemoattractants, PARC (pulmonary and activation regulated chemokine), macrophage derived chemokine (MDC), and I-309, the MDC receptor CCR4, and T lymphocyte activation markers, CD25, CD26, CD62L, CD71, and CD30, and to correlate them with the counts of CD3+ T lymphocytes in the conjunctiva of patients with VKC.
Method: Conjunctival biopsy specimens from 11 patients with active VKC, and eight control subjects were studied by immunohistochemical techniques using a panel of monoclonal and polyclonal antibodies directed against PARC, MDC, I-309, CCR4, CD25, CD26, CD62L, CD71, and CD30. The numbers of positively stained cells were counted. The phenotype of inflammatory cells expressing chemokines was examined by double immunohistochemistry.
Results: In the normal conjunctiva, vascular endothelial cells in the upper substantia propria showed weak immunoreactivity for CD26. There was no immunoreactivity for the other antibodies. VKC specimens showed inflammatory cells expressing PARC, MDC, and I-309. The numbers of PARC+ inflammatory cells were higher than the numbers of MDC+ and I-309+ inflammatory cells and the mean values of the three groups differed significantly (17.0 (SD 10.1); 9.5 (9.9), and 4.3 (7.9), respectively, p = 0.0117, ANOVA). The numbers of PARC+ inflammatory cells had the strongest correlation with the numbers of CD3+ T lymphocytes. Few CCR4+ inflammatory cells were observed in only three specimens. Double immunohistochemistry revealed that all inflammatory cells expressing chemokines were CD68+ monocytes/macrophages. The numbers of CD25+ T lymphocytes were higher than the numbers of CD26+, CD62L+, CD71+, and CD30+ T lymphocytes and the mean values of the five groups differed significantly (46.2 (27.9), 30.7 (16.0), 20.1 (8.6), 7.8 (7.7), and 6.5 (4.0), respectively, p <0.001, ANOVA). The numbers of CD25+ T lymphocytes had the strongest correlation with the numbers of CD3+ T lymphocytes.
Conclusion: These results suggest a potential role for PARC, MDC, and I-309 in attracting T lymphocytes into conjunctiva in VKC. T lymphocytes in VKC are activated and express several activation markers which might contribute to the pathogenesis of VKC.
Keywords: conjunctiva, vernal keratoconjunctivitis, allergy, chemokines, T lymphocyte activation
Vernal keratoconjunctivitis (VKC) is an allergic chronic seasonally exacerbated bilateral external ocular inflammation that primarily affects children and young adults. The disease is characterised by recurrent symptoms of severe itching, photophobia, lacrimation, and discharge. The histopathology of VKC is characterised by infiltration of the conjunctiva by mononuclear cells, mainly consisting of CD4+ T helper (Th) cells.1 During the development of an immune response, naive resting CD4+ T cells are polarised to either Th1 or Th2 phenotype defined by the pattern of cytokines they produce.2 Inflammation associated with allergic disorders is regarded as a Th2 dominant immune response. Th2 cells are known to produce interleukin (IL) 3, IL-4, IL-5, IL-10, IL-13, and granulocyte-macrophage colony stimulating factor, which are involved in B cell switching to immunoglobulin E, mast cell proliferation, and eosinophil activation and recruitment.3 Recently, several studies demonstrated that CD4+ T lymphocytes in VKC expressed Th2-type cytokines.4,5,6
Despite the clear evidence for T cell involvement in VKC, the mechanisms of T cell recruitment within the conjunctiva remain poorly defined. Trafficking of activated T cells into inflammatory sites is a tightly controlled process directed by multiple molecules in particular adhesion molecules and chemokines.7,8 In a previous study, we demonstrated an increase in the expression of adhesion molecules in the conjunctiva from patients with VKC compared to control subjects.1 Chemokines are a superfamily of chemoattractant peptides involved in normal leucocyte trafficking and recruitment during inflammation. Chemokines are grouped into the CXC, CC, C, and CX3C subfamilies on the basis of the arrangement of the conserved cysteine residues. The specific effects of chemokines are mediated by a family of seven transmembrane spanning G protein coupled receptors.9 In previous reports, we demonstrated an increase in the expression of RANTES (regulated upon activation, normal T cell expressed and secreted), eotaxin, monocyte chemotactic protein (MCP)-1, and MCP-3,10 and abundant expression of the chemokine receptor CXCR3 on T lymphocytes11 in the conjunctiva of patients with active VKC.
A number of novel CC chemokines have been described recently, which are potent chemoattractants for T lymphocytes: PARC (pulmonary and activation regulated chemokine), macrophage derived chemokine (MDC), and I-309. PARC shows a specificity for naive resting T lymphocytes. The receptor for PARC has not yet been identified.12 MDC, and I-309 preferentially attract activated Th2 lymphocytes by interacting with CCR4 and CCR8 receptors, respectively.13,14 Given the fact that T cells are present in increased numbers in the conjunctiva from patients with VKC and their presence is thought to be caused by active recruitment, we investigated the expression of these new T lymphocyte specific chemoattractants in VKC, and the cellular source of these chemokines. In addition, we examined the expression of T cell activation markers, CD25 (the IL-2 receptor α-chain), CD26 or dipeptidyl peptidase IV (DPP IV), CD62L (l-selectin), CD71 (transferrin receptor), and CD30. Furthermore, we examined the correlations between the numbers of T lymphocytes and the numbers of inflammatory cells expressing these CC chemokines and activation markers.
PATIENTS AND METHODS
Patients
Eleven consecutive Saudi patients with active VKC seen at the outpatient clinic of King Abdulaziz University Hospital were included in the study. The patients were seven males and four females. The mean age was 12.6 (SD 2.3) years (range 8–15 years). The symptoms mentioned by all the patients were itching, redness, photophobia, and tearing. Each patient underwent complete ophthalmic examination, and the corneal and conjunctival changes were noted and recorded. All patients had the limbal form of the disease characterised by broad gelatinous infiltrates of the limbus. Limbal conjunctival biopsy specimens were obtained from each patient. None of the patients was on topical therapy before obtaining the biopsy. In addition, eight limbal conjunctival biopsy specimens were obtained from patients undergoing strabismus surgery without obvious inflammation and served as controls. None of the controls had a history or signs of VKC. The controls were from the same age group, and were five males and three females. All the controls were Saudi, and were operated in King Abdulaziz University Hospital. This study was approved by the Research Center, College of Medicine, King Saud University, and the patients admitted to the study gave their informed consent.
Immunohistochemical staining
The conjunctival biopsy specimens were immediately snap frozen in Tissue-Tek optimum cutting temperature (OCT) compound (Miles Laboratories, IN, USA) and maintained at −80°C until use. For immunohistochemistry, 5 μm serially cut cryostat sections were dried overnight at room temperature, fixed in absolute acetone for 10 minutes, and then treated with 2% hydrogen peroxide in methanol for 3 minutes to block endogenous peroxidase activity. After rinsing three times in phosphate buffered saline (PBS) at pH 7.2 for 15 minutes, the slides were incubated for 30 minutes with the monoclonal and polyclonal antibodies listed in Table 1. Optimal concentrations of all antibodies used were determined in pilot experiments. After a wash with PBS, the sections were incubated for 30 minutes with Envision+, Peroxidase, Rabbit, or EnVision+, Peroxidase, Mouse (Dako, CA, USA). These are goat anti-rabbit or anti-mouse immunoglobulins conjugated to peroxidase labelled dextran polymer. The products react with rabbit immunoglobulins or with mouse immunoglobulins of all classes and minimally with human immunoglobulins, thus allowing better visualisation. The slides were washed again with PBS and the reaction product was visualised by incubation for 10 minutes in 0.05M acetate buffer at pH 4.9, containing 0.05% 3-amino-9-ethylcarbazole (Sigma-Aldrich, Bornem, Belgium) and 0.01% hydrogen peroxide, resulting in bright red immunoreactive sites. The slides were faintly counterstained with Harris haematoxylin. Finally, the sections were rinsed with distilled water and coverslipped with glycerol. Omission or substitution of the primary antibody with an irrelevant antibody of the same species was used as a negative control. Positive controls were previously positive staining tissues. As positive controls, we used samples from other tissues, including small intestine from patients with Crohn’s disease, synovia from patients with rheumatoid arthritis, liver, and lymph nodes, showing a positive staining reaction with the same set of antibodies as that used in the present study.
Table 1.
Monoclonal and polyclonal antibodies used in this study
| Primary antibody | Dilution | Source |
| Anti-CD3 (UCHT1) (mc) | 1:400 | Dako |
| Anti-PARC (64507) (mc) | 1:50 | R & D Systems |
| Anti-PARC (pc) | 1:50 | Pepro Tech Inc |
| Anti-MDC (pc) | 1:10 | Pepro Tech Inc |
| Anti-I-309 (35305.11) (mc) | 1:50 | R & D Systems |
| Anti-CCR4 (H-48) (pc) | 1:20 | Santa Cruz Biotechnology, Inc |
| Anti-CD25 (ACT-1) (mc) | 1:3 | Dako |
| Anti-CD26 (M-A261) (mc) | 1:50 | PharMingen |
| Anti-CD62L (M7084) (mc) | 1:10 | Dako |
| Anti-CD71 (M-A712) (mc) | 1:100 | PharMingen |
| Anti-CD30 (Ber-H2) (mc) | 1:10 | Dako |
| Anti-CD30 (Ki-1) (mc) | 1:10 | Dako |
Location of manufacturers: Dako, CA, USA; R & D Systems Europe Ltd, Abingdon, UK; PeproTech Inc, Rocky Hill, NJ, USA; Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA; PharMingen, San Diego, CA, USA.
PARC = pulmonary and activation regulated chemokine; MDC = macrophage derived chemokine.
Double immunohistochemistry
To examine the phenotype of inflammatory cells expressing chemokines, cryostat sections were studied by sequential double immunohistochemistry. Co-localisation studies were performed in four VKC specimens. After rinsing the slides with PBS, they were incubated for 30 minutes with the monoclonal antibody to determine the cellular phenotype (CD68 (KP1); 1:1000, monocytes/macrophages, Dakopatts A/S, Copenhagen, Denmark) and rinsed again in PBS. Subsequently, the sections were incubated for 30 minutes with Envision+, Peroxidase, Mouse (Dako, CA, USA) and washed again with PBS. Then, the reaction product was visualised by incubation for 10 minutes in 0.05M acetate buffer at pH 4.9, containing 0.05% 3-amino-9-ethylcarbazole and 0.01% hydrogen peroxide, resulting in red immunoreactive staining. Afterwards the sections were rinsed with PBS, washed with distilled water and incubated for 30 minutes with the chemokine antibodies. After a wash with PBS, the sections were incubated for 30 minutes with a rabbit anti-mouse alkaline phosphatase labelled antibody (Sigma-Aldrich). The blue reaction product was developed using fast blue BB salt (4-benzoylamino-2.5-diethoxybenzene-diazonium chloride) (Sigma-Aldrich) for 5 minutes.
Quantitation
Cells were counted in five representative fields that were chosen on the basis of the presence of an adequate number of inflammatory cells. We ignored fields in which no positively stained cells were present. We used an eye piece calibrated grid with 40× magnification. With this magnification and calibration, we counted the cells present in an area of 0.33 × 0.22 mm.
Statistical analysis
All data are presented as mean (SD). The data were analysed using one way analysis of variance (ANOVA), non-parametric one way ANOVA based on the Kruskal-Wallis test, correlation analysis, and ridge regression. Programs 4R, 7D, and 3S from the BMDP Statistical Package were used. The differences were considered significant if the p value was <0.05.
RESULTS
There was no staining in the negative control slides. In normal conjunctiva, weak CD26 immunoreactivity was observed on vascular endothelial cells in the upper substantia propria. There was no immunoreactivity for PARC, MDC, I-309, CCR4, CD25, CD62L, CD71, and CD30.
In VKC specimens, a heavy inflammatory infiltrate of CD3+ T lymphocytes was noted in the epithelium and in the substantia propria just beneath the epithelium in all specimens. Inflammatory mononuclear cells expressing granular cytoplasmic PARC, MDC, and I-309 were noted in the upper substantia propria at sites of heavy CD3+ T lymphocyte accumulation (Fig 1). PARC+ inflammatory cells were noted in all specimens, whereas MDC+ inflammatory cells were observed in nine specimens and I-309+ inflammatory cells were observed in six specimens. Both PARC antibodies that we used generated the same labelling pattern on inflammatory cells. Membranous immunoreactivity for CCR4 was noted on inflammatory mononuclear cells in the upper substantia propria in three specimens.
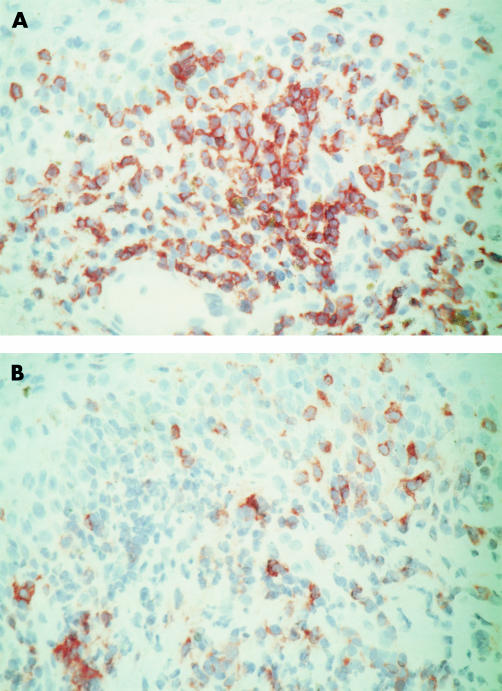
Figure 1.
Vernal keratoconjunctivitis. Serial sections illustrating immunohistochemical stainings for CD3 (A) PARC (B) showing PARC+ inflammatory mononuclear cells at sites of heavy CD3+ T lymphocyte accumulation in the upper substantia propria (original magnification ×40).
Double immunohistochemistry to confirm the phenotype of chemokine positive inflammatory cells showed that all the mononuclear cells expressing chemokines were CD68 positive monocytes/macrophages (Fig 2). Many of the PARC+ inflammatory cells had a dendritic morphology.
Figure 2.

Vernal keratoconjunctivitis. Double immunohistochemical staining for PARC (blue), and CD68 (red) showing PARC positive cells coexpressing CD68 marker (arrows) (original magnification ×100).
The numbers of inflammatory cells expressing PARC were higher than the numbers of inflammatory cells expressing MDC and I-309 (Table 2). The mean values of the three groups differed significantly (p = 0.0117, ANOVA), Furthermore, post-ANOVA pairwise comparisons based on the Bonferroni method showed that the numbers of inflammatory cells expressing PARC were significantly higher than the numbers of inflammatory cells expressing I-309 (p = 0.0032, t test).
Table 2.
Numbers* of immune cells in VKC specimens (n=11)
| Cell type | Mean (SD) | Range | No of specimens with detectable expression |
| CD3 | 184.1 (55.3) | 110–280 | 11 |
| PARC | 17.0 (10.1) | 6–33 | 11 |
| MDC | 9.5 (9.9) | 0–30 | 9 |
| I-309 | 4.3 (7.9) | 0–21 | 6 |
| CCR4 | 2.8 (5.7) | 0–16 | 3 |
| CD25 | 46.2 (27.9) | 12–90 | 11 |
| CD26 | 30.7 (16.0) | 14–42 | 11 |
| CD62L | 20.1 (8.6) | 5–35 | 11 |
| CD71 | 7.8 (7.7) | 0–18 | 7 |
| CD30 | 6.5 (4.0) | 3–15 | 11 |
VKC = vernal keratoconjunctivitis; PARC = pulmonary and activation regulated chemokine; MDC = macrophage derived chemokine.
*Cells counted in an area of 0.33 × 0.22 mm.
Membranous immunoreactivity for CD25, CD26, CD62L, CD71, and CD30 was noted on mononuclear inflammatory cells in the epithelial and stromal inflammatory infiltrate (Fig 3). Inflammatory cells expressing CD25, CD26, CD62L, and CD30 were noted in all specimens, whereas CD71+ cells were observed in seven specimens. A marked upregulation of CD26 expression was noted on superficial and deep stromal vascular endothelial cells (Fig 4). CD30 expression was confirmed by staining with two anti-CD30 monoclonal antibodies. The activation markers and CCR4 were shown to be expressed on CD3+ T lymphocytes by serial sections (Fig 3).
Figure 3.
Vernal keratoconjunctivitis. Serial sections illustrating immunohistochemical stainings for CD3 (A) and CD25 (B) showing CD3+ CD25+ T lymphocytes in the epithelial and stromal inflammatory infiltrate (original magnification ×40).
Figure 4.
Vernal keratoconjunctivitis. Immunohistochemical staining for CD26 showing membranous immunoreactivity on mononuclear inflammatory cells (arrows) and on the vascular endothelium (arrowheads) (original magnification ×40).
The numbers of inflammatory cells expressing CD25 were higher than the numbers of inflammatory cells expressing CD26, CD62L, CD71, and CD30 (Table 2). The mean values of the five groups differed significantly (p <0.001, ANOVA done by non-parametric Kruskal-Wallis test). Furthermore, post-ANOVA pairwise comparisons showed that the numbers of inflammatory cells expressing CD25 were significantly higher than the numbers of inflammatory cells expressing CD71 (Z* = 4.24) and CD30 (Z = 4.44). The numbers of inflammatory cells expressing CD26 were significantly higher than the numbers of inflammatory cells expressing CD71 (Z = 3.59) and CD30 (Z = 3.79). (*The critical Z value was Z ≥2.81 for five groups at a 5% level of significance (Kruskal-Wallis test).)
Correlations between the numbers of CD3+ T lymphocytes and the numbers of inflammatory cells expressing chemokines
A significant positive correlation was observed between the numbers of CD3+ T lymphocytes and the numbers of inflammatory cells expressing PARC (r = 0.6709, p = 0.0238), MDC (r = 0.7453, p = 0.0085), and I-309 (r = 0.6389, p = 0.0343). Ridge regression analysis indicated that the numbers of inflammatory cells expressing PARC had the strongest correlation (79% explained variation) with the numbers of CD3+ T lymphocytes.
Correlations between the numbers of CD3+ T lymphocytes and the numbers of inflammatory cells expressing activation markers
A significant positive correlation was observed between the numbers of inflammatory cells expressing CD3+ T lymphocytes and the numbers of inflammatory cells expressing CD25 (r = 0.7789, p = 0.0047), CD62L (r = 0.6464, p = 0.0316), and CD71 (r = 0.6259, p = 0.0394). Ridge regression analysis indicated that the numbers of inflammatory cells expressing CD25 had the strongest correlation (46.1% explained variation) with the numbers of CD3+ T lymphocytes.
Correlations between the numbers of inflammatory cells expressing chemokines and the numbers of inflammatory cells expressing activation markers
A significant positive correlation was observed between the numbers of inflammatory cells expressing PARC and the numbers of inflammatory cells expressing CD25 (r = 0.8782, p = 0.0004). In addition, there was a significant positive correlation between the numbers of inflammatory cells expressing MDC and the numbers of inflammatory cells expressing CD26 (r = 0.6354, p = 0.0357).
DISCUSSION
In vitro, PARC mRNA is expressed by monocyte derived dendritic cells, and activated monocytes.12,15–19 PARC preferentially attracts naive resting T cells (CD45RA+).12 However, Hieshima et al16 demonstrated that PARC is chemotactic for both activated (CD3+) T cells and non-activated (CD14−) lymphocytes. PARC binds to a yet unknown receptor expressed on naive T cells.12,16 The specific expression of PARC by dendritic cells at the site of initiation of an immune response, combined with its chemotactic activity for naive T cells, suggests that PARC has an important role in the induction of immune responses.12
In the present study, we detected cytoplasmic expression of PARC by CD68 positive monocytes/macrophages. Many of these cells had a dendritic morphology. In agreement with our data, previous studies showed that PARC mRNA was restricted to CD68 positive macrophages in human atherosclerotic plaques.15 In addition, high PARC expression was noted in alveolar macrophages and dendritic cells in the germinal centres of regional lymph nodes.12,16,19
MDC is constitutively produced by dendritic cells, macrophages, and thymic medullary epithelial cells, whereas monocytes, B lymphocytes, natural killer cells, and CD4+ T lymphocytes produce MDC only upon appropriate stimulation.20–25 More recently, MDC was shown to be preferentially produced by activated Th2 cells.26 MDC is chemotactic for activated T lymphocytes, IL-2 activated natural killer cells, and monocyte derived dendrtic cells.20,21 Subsequently, MDC was shown to act selectively on chronically activated Th2 lymphocytes by interacting with the CCR4 receptor,13 which is preferentially expressed by this T cell effector subset.27,28 More recently, MDC was also found to be able to induce human eosinophil chemotaxis in a CCR3 and CCR4 independent manner.29
The conjunctiva from patients with VKC showed cytoplasmic expression of MDC, and I-309 by CD68 positive monocytes/macrophages. Previous studies have reported expression of MDC by dendritic cells in skin biopsy specimens from patients with atopic dermatitis, a Th2 oriented disorder.25,26 In a mouse model of allergic inflammation in the lung, MDC is produced by alveolar macrophages.30 In this animal model, Lloyd et al31 showed that the CCR4/MDC pathway has a dominant role in effector Th2 recruitment under conditions of chronic, repeated antigen stimulation. The expression of I-309 by monocytes/macrophages in the conjunctiva from patients with VKC is consistent with in vitro studies that demonstrated I-309 expression and secretion by appropriately stimulated human peripheral blood monocytes.32 Surprisingly, few T cells in VKC conjunctiva expressed CCR4. Our finding of poor CCR4 expression might be due to CCR4 internalisation after stimulation with its ligands.33,34
The expression of PARC, and MDC by monocytes/macrophages in VKC conjunctiva might be explained by upregulation by inflammatory cytokines. Recently, several studies demonstrated that the Th2 cytokines, IL-4, and IL-13 induced PARC expression in macrophages19 and MDC expression in monocytes.24,35
In this study, we found that the T cells infiltrating the conjunctiva of patients with VKC express several activation markers. CD25+ cells were the most numerous followed by CD26+ cells and CD62L+ cells. CD71+ cells and CD30+ cells were few. In addition, the numbers of CD25+ cells had the strongest correlation with the numbers of CD3+ T lymphocytes. T cells express CD25 upon activation36 and CD25 expression is promoted by the Th2 cytokine, IL-4.37 Stimulation of CD4+ T cells secreting Th2 type cytokines by dendritic cells elicits T cell proliferation, production of cytokines, and upregulates CD25 expression.38 Recently, Suto et al39 demonstrated that CD4+ CD25+ T cells modulate the Th1 and Th2 cell balance towards Th2 cells and thus upregulate Th2 cell mediated allergic inflammation in the airways. Our findings are in agreement with previous studies in other allergic disorders.40–43
CD26 is a cell surface protease constitutively expressed on a wide variety of epithelial, endothelial, and lymphoid cell types. It is present on resting T cells, and T cell activation is accompanied by enhanced expression of CD26. CD26 has been shown to have a role in T cell activation and T cell mediated immunity.44–46 The conjunctiva from patients with VKC showed strong expression of CD26 on T cells and vascular endothelial cells. Similar expression was noted in the human bronchus.47 In vitro studies demonstrated that CD4+ memory T cells with the capacity for transendothelial migration express CD26 and CD25 and that CD26 is one likely candidate for control of T cell transmigration.48 It is suggested that CD26 on blood vessels might be involved in the processing of intravascular peptides, such as substance P and in the regulation of substance P induced plasma leakage.47
Recently, several studies demonstrated that CD26 can process several chemokines such as eotaxin, RANTES, and MDC.23,49,50 After cleavage of eotaxin by CD26, its chemotactic potency for blood eosinophils and its signalling capacity through the CCR3 receptor were reduced.49 Processed RANTES is inactive in chemotaxis of purified monocytes and enhances chemotactic migration of T cells.50 Truncated MDC does not interact with CCR4 and does not attract Th2 cells.23 Recently, Wilheim et al51 demonstrated that CD26 expression is correlated with a Th1-like phenotype. Therefore, CD26 expressed mainly on Th1 cells may represent a negative feedback for allergic reactions mediated by CC chemokines.
Del Prete et al52 have shown that CD30, a member of the tumour necrosis factor/nerve growth factor receptor superfamily, is preferentially expressed by human T cell clones producing Th2 cytokines. In contrast, Hamann et al53 demonstrated that CD30 can be expressed by Th1-like cells, contributing to interferon γ secretion. Furthermore, Alzona et al54 found that CD30 defines a subset of activated T cells that can produce both interferon γ and IL-5 and exhibit potent activity for B cell immunoglobulin production. Evidence for in vivo involvement of CD30+ cells in allergic disease has appeared from immunohistochemical finding of CD30+ cells in acute atopic dermatitis biopsies. On the other hand, no significant CD30 expression could be found in subacute/chronic atopic dermatitis lesions or in any of the specimens of allergic contact dermatitis.55 In the present study, CD30 expression by a few T cells in the conjunctiva of patients with VKC was confirmed by staining with two anti-CD30 monoclonal antibodies (Ber-H2, and Ki-1). Similarly, CD71, a marker expressed by activated proliferating T cells,56 was expressed by a few T cells.
CD62L is a cell surface glycoprotein present on most circulating human lymphocytes. It is mainly involved in the adhesion of T cells to the high endothelial venules of peripheral lymph nodes and subsequent extravasation in peripheral lymph nodes. In addition, it is suggested that CD62L is also crucial for migration of lymphocytes to sites of inflammation. Lymphocytes shed CD62L upon activation, but can be re-expressed upon return to the resting state.57 The conjunctiva from patients with VKC contained relatively few CD62L+ lymphocytes. Our observations are consistent with previous data in inflamed skin, appendix, and synovium,58 and in the airways of a murine model of allergic asthma.59 These findings are consistent with shedding of CD62L upon activation.58 Interestingly, Kanegane et al60 demonstrated that CD62L positive human memory CD4+ T cells produce mainly Th2 cytokines, whereas CD62L negative CD4+ T cells produce mainly Th1 cytokines.
In conclusion, PARC and MDC expressed by CD68 positive monocytes/macrophages may function to recruit naive and activated T lymphocytes in VKC. PARC could play a major part in the recruitment of naive T cells which might initiate the immune responses. PARC and MDC might further attract activated T cells. T lymphocytes infiltrating the conjunctiva of patients with VKC express several activation markers which might contribute to the pathogenesis of VKC.
Acknowledgments
This work is supported in part by the Fund for Scientific Research of Flanders (FWO-Vlaanderen). Sofie Struyf is a research assistant of the FWO-Vlaanderen.
The authors thank Ms Christel Van den Broeck and Ms Martin Verhoeven for technical assistance, Mr Dustan Kangave for statistical assistance, and Ms Connie B Unisa-Marfil for secretarial work.
REFERENCES
- 1.Abu El-Asrar AM, Geboes K, Al-Kharashi S, et al. Adhesion molecules in vernal keratoconjunctivitis. Br J Ophthalmol 1997;81:1099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 1996;17:138–46. [DOI] [PubMed] [Google Scholar]
- 3.Umetsu DT, De Kruyff RH. TH1 and TH2 CD4+ cells in human allergic diseases. J Allergy Clin Immunol 1997;100:1–6. [DOI] [PubMed] [Google Scholar]
- 4.Calder VL, Jolly G, Hingorani M, et al. Cytokine production and mRNA expression by conjunctival T-cell lines in chronic allergic eye disease. Clin Exp Allergy 1999;29:1214–22. [DOI] [PubMed] [Google Scholar]
- 5.Metz DP, Hingorani M, Calder VL, et al. T-cell cytokines in chronic allergic eye disease. J Allergy Clin Immunol 1997;100:817–24. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi A, De Franchis G, Zancanaro F, et al. Identification of local Th2 and Th0 lymphocytes in vernal conjunctivitis by cytokine flow cytometry. Invest Ophthalmol Vis Sci 1999;40:3036–40. [PubMed] [Google Scholar]
- 7.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science 1996;272:60–6. [DOI] [PubMed] [Google Scholar]
- 8.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol 2000;18:217–42. [DOI] [PubMed] [Google Scholar]
- 9.Murphy PM, Baggiolini M, Charo IF, et al. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 2000;52:145–76. [PubMed] [Google Scholar]
- 10.Abu El-Asrar AM, Struyf S, Al-Kharashi SA, et al. Chemokines in the limbal form of vernal keratoconjunctivitis. Br J Ophthalmol 2000;84:1360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu El-Asrar AM, Struyf S, Al Mosallam AA, et al. Expression of chemokine receptors in vernal keratoconjunctivitis. Br J Ophthalmol 2001;85:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adema GJ, Hartgers F, Verstraten R, et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature 1997;387:713–17.9192897 [Google Scholar]
- 13.Imai T, Chantry D, Raport CJ, et al. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem 1998;273;1764–6. [DOI] [PubMed] [Google Scholar]
- 14.Zingoni A, Soto H, Hedrick JA, et al. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol 1998;161:547–51. [PubMed] [Google Scholar]
- 15.Reape TJ, Rayner K, Manning CD, et al. Expression and cellular localization of the CC chemokines PARC and ELC in human atherosclerotic plaques. Am J Pathol 1999;154:365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hieshima K, Imai T, Baba M, et al. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1α/LD78α and chemotactic for T lymphocytes, but not for monocytes. J Immunol 1997;159:1140–9. [PubMed] [Google Scholar]
- 17.Sallusto F, Palermo B, Lenig D, et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol 1999;29:1617–25. [DOI] [PubMed] [Google Scholar]
- 18.Vissers JLM, Hartgers FC, Lindhout E, et al. Quantitative analysis of chemokine expression by dendritic cell subsets in vitro and in vivo. J Leukoc Biol 2001;69:785–93. [PubMed] [Google Scholar]
- 19.Kodelja V, Müller C, Politz O, et al. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1α with a Th2-associated expression pattern. J Immunol 1998;160:1411–18. [PubMed] [Google Scholar]
- 20.Chang M, McNinch J, Elias C III, et al. Molecular cloning and functional characterization of a novel CC chemokine, stimulated T cell chemotactic protein (STCP-1) that specifically acts on activated T lymphocytes. J Biol Chem 1997;272:25229–37. [DOI] [PubMed] [Google Scholar]
- 21.Godiska R, Chantry D, Raport CJ, et al. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med 1997;185:1595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaniel C, Pardali E, Sallusto F, et al. Activated murine B lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J Exp Med 1998;188:451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A, Gray PA, Van Damme J, et al. Macrophage-derived chemokine. J Leukoc Biol 2000;68:400–4. [PubMed] [Google Scholar]
- 24.Andrew DP, Chang MS, McNinch J, et al. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol 1998;161:5027–38. [PubMed] [Google Scholar]
- 25.Vulcano M, Albanesi C, Stoppacciaro A, et al. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur J Immunol 2001;31:812–22. [DOI] [PubMed] [Google Scholar]
- 26.Galli G, Chantry D, Annunziato F, et al. Macrophage-derived chemokine production by activated human T cells in vitro and in vivo: preferential association with the production of type 2 cytokines. Eur J Immunol 2000;30:204–10. [DOI] [PubMed] [Google Scholar]
- 27.D’Ambrosio D, Iellem A, Bonecchi R, et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol 1998;161:5111–15. [PubMed] [Google Scholar]
- 28.Imai T, Nagira M, Takagi S, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokine thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol 1999;11:81–8. [DOI] [PubMed] [Google Scholar]
- 29.Bochner BS, Bickel CA, Taylor ML, et al. Macrophage-derived chemokine induces human eosinophil chemotaxis in a CC chemokine receptor 3-and CC chemokine receptor 4-independent manner. J Allergy Clin Immunol 1999;103:527–32. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalo JA, Pan Y, Lloyd CM, et al. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol 1999;163: 400–11. [PubMed] [Google Scholar]
- 31.Lloyd CM, Delaney T, Nguyen T, et al. CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/ monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med 2000;191:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selvan RS, Zhou LJ, Krangel MS. Regulation of I-309 gene expression in human monocytes by endogenous interleukin-1. Eur J Immunol 1997;27:687–94. [DOI] [PubMed] [Google Scholar]
- 33.Inngjerdinger M, Damaj B, Maghazachi AA. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J Immunol 2000;164: 4048–54. [DOI] [PubMed] [Google Scholar]
- 34.de Lavareille A, Roufosse F, Schandene L, et al. Clonal Th2 cells associated with chronic hypereosinophilia: TARC-induced CCR4 down-regulation in vivo. Eur J Immunol 2001;31:1037–46. [DOI] [PubMed] [Google Scholar]
- 35.Bonecchi R. Sozzani S, Stine JT, et al. Divergent effects of interleukin-4 and interferon- γ on macrophage-derived chemokine production: an amplification circuit of polarized T helper 2 responses. Blood 1998;92:2668–71. [PubMed] [Google Scholar]
- 36.Lai KN, Leung JCK, Lai FM. Soluble interleukin 2 receptor release, interleukin 2 production, and interleukin 2 receptor expression in activated T lymphocytes in vitro. Pathology 1991;23:224–8. [DOI] [PubMed] [Google Scholar]
- 37.Butcher RDJ, Cushley W. Flow cytometric studies of IL-4 stimulated expression of CD25 antigen by quiescent human B lymphocyte subpopulations. Immunology 1991;74:511–18. [PMC free article] [PubMed] [Google Scholar]
- 38.Roufosse F, Schandene L, Sibille C, et al. T-cell receptor-independent activation of clonal Th2 cells associated with chronic hypereosinophilia. Blood 1999;94:994–1002. [PubMed] [Google Scholar]
- 39.Suto A, Nakajima H, Kagami SI, et al. Role of CD4+ CD25+ regulatory T cells in T helper 2 cell-mediated allergic inflammation in the airways. J Respir Crit Care Med 2001;164:680–7. [DOI] [PubMed] [Google Scholar]
- 40.Hoshino M, Nakamura Y, Shin Z, et al. Effects of Ketotifen on symptoms and on bronchial mucosa in patients with atopic asthma. Allergy 1997;52:814–20. [DOI] [PubMed] [Google Scholar]
- 41.Bentley AM, Meng Q, Robinson DS, et al. Increases in activated T lymphocytes, eosinophils, and cytokine mRNA expression for interleukin-5 and granulocyte/macrophage colony-stimulating factor in bronchial biopsies after allergen inhalation challenge in atopic asthmatics. Am J Respir Cell Mol Biol 1993;8:35–42. [DOI] [PubMed] [Google Scholar]
- 42.Igarashi Y, Goldrich MS, Kaliner MA, et al. Quantitation of inflammatory cells in the nasal mucosa of patients with allergic rhinitis and normal subjects. J Allergy Clin Immunol 1995;95:716–25. [DOI] [PubMed] [Google Scholar]
- 43.Calderon MA, Lozewicz S, Prior A, et al. Lymphocyte infiltration and thickness of the nasal mucous membrane in perennial and seasonal allergic rhinitis. J Allergy Clin Immunol 1994;93:635–43. [DOI] [PubMed] [Google Scholar]
- 44.Fleischer B. CD26: a surface protease involved in T-cell activation. Immunol Today 1994;15:180–4. [DOI] [PubMed] [Google Scholar]
- 45.De Meester I, Korom S, Van Damme J, et al. CD26, let it cut or cut it down. Immunol Today 1999;20:367–75. [DOI] [PubMed] [Google Scholar]
- 46.Steinbrecher A, Reinhold D, Quigley L, et al. Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up-regulates TGF-β1 secretion in vivo. J Immunol 2001;166:2041–8. [DOI] [PubMed] [Google Scholar]
- 47.Van der Velden VHJ, Wierenga-Wolf AF, Adriaansen-Soeting PWC, et al. Expression of aminopeptidase N and dipeptidyl peptidase IV in the healthy and asthmatic bronchus. Clin Exp Allergy 1998;28:110–20. [DOI] [PubMed] [Google Scholar]
- 48.Masuyama JI, Berman JS, Cruikshank WW, et al. Evidence for recent as well as long term activation of T cells migrating through endothelial cell monolayers in vitro. J Immunol 1992;148:1367–74. [PubMed] [Google Scholar]
- 49.Struyf S, Proost P, Schols D, et al. CD26/dipeptidyl-peptidase IV down-regulates the eosinophil chemotactic potency, but not the anti-HIV activity of human eotaxin by affecting its interaction with CC chemokine receptor 3. J Immunol 1999;162:4903–9. [PubMed] [Google Scholar]
- 50.Iwata S, Yamaguchi N, Munakata Y, et al. CD26/dipeptidyl peptidase IV differentially regulates the chemotaxis of T cells and monocytes toward RANTES: possible mechanism for the switch from innate to acquired immune response. Int Immunol 1999;11:417–26. [DOI] [PubMed] [Google Scholar]
- 51.Wilheim M, Ebner C, Baier K, et al. Cell surface characterization of T lymphocytes and allergen-specific T cell clones: correlation of CD26 expression with TH1 subsets. J Allergy Clin Immunol 1997;100:348–55. [DOI] [PubMed] [Google Scholar]
- 52.Del Prete G, De Carli M, D’Elios MM, et al. CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J Exp med 1995;182:1655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamann D, Hilkens CMU, Grogan JL, et al. CD30 expression does not discriminate between human Th1- and Th2-type T cells. J Immunol 1996;156: 1387–91. [PubMed] [Google Scholar]
- 54.Alzona M, Jack HM, Fisher RI, et al. CD30 defines a subset of activated human T cells that produce IFN-γ and IL-5 and exhibit enhanced B cell helper activity. J Immunol 1994;153:2861–7. [PubMed] [Google Scholar]
- 55.Dummer W, Rose C, Brocker EB. Expression of CD30 on T helper cells in the inflammatory infiltrate of acute atopic dermatitis but not of allergic contact dermatitis. Arch Dermatol Res 1998;290-598–602. [DOI] [PubMed] [Google Scholar]
- 56.Salmon M, Bacon PA, Symmons DP, et al. Transferrin receptor expression by stimulated cells in mixed lymphocyte culture. Immunology 1985;54:559–64. [PMC free article] [PubMed] [Google Scholar]
- 57.Gallatin M, St John TP, Siegelman M, et al. Lymphocyte homing receptors. Cell 1986;44:673–80. [DOI] [PubMed] [Google Scholar]
- 58.Munro JM, Briscoe DM, Tedder TF. Differential regulation of leucocyte L-selectin (CD62L) expression in normal lymphoid and inflamed extralymphoid tissues. J Clin Pathol 1996;49:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilder JA, Collie DDS, Bice DE, et al. Ovalbumin aerosols induce airway hyperreactivity in naive DO11.10 T cell receptor transgenic mice without pulmonary eosinophilia or OVA-specific antibody. J Leukoc Biol 2001;69:538–47. [PubMed] [Google Scholar]
- 60.Kanegane H, Kasahara Y, Niida Y, et al. Expression of L-selectin (CD62L) discriminates Th1- and Th2-like cytokine-producing memory CD4+ T cells. Immunology 1996;87:186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]