Abstract
Aims: To prospectively analyse the present incidence of ROP (retinopathy of prematurity) in a well defined geographical area in Sweden, and to compare it with that from a decade earlier in exactly the same area.
Methods: Infants born between 1 August 1998 and 31 July 2000 with a birth weight of 1500 g or less were studied. They were screened for ROP from 5 weeks of postnatal age until the retina was entirely vascularised. The incidence of ROP, with its various stages, was compared with that of a previous (1988–90) population based study in the same geographical area.
Results: The incidence of ROP in the present study was 36.4% (mild (18.2%) and severe ROP (18.2%)), which was similar to that of the previous study. Gestational age at birth remained the most important risk factor for ROP. We found a change in the distribution of ROP. The probability of ROP, particularly severe ROP, was highest in the most immature infants while it was lower in the least immature ones.
Conclusions: The incidence of ROP remained the same in two consecutive population based studies. The more “mature” infants developed ROP, particularly severe ROP, less often, while the most immature infants had ROP more often, particularly severe ROP.
Keywords: retinopathy of prematurity, Sweden
Improvements in neonatal care continue to increase survival of prematurely born infants. It is therefore of interest to perform consecutive, epidemiological studies to investigate whether the incidence of ROP is changing in the population.
In the Stockholm area of Sweden, a population based study of the incidence and natural course of ROP was previously performed in infants with a birth weight of 1500 g or less, born 1988–90. Of 260 infants, 40% developed ROP (20% mild and 20% severe)1 and 10.8% were cryotreated (at a slightly earlier stage than in the Cryo ROP study).2 The present study aimed to analyse the incidence of ROP again, 10 years later, in exactly the same geographical area, and to investigate whether the presumed increase in survival rate and improved neonatal care had affected the incidence and the distribution of ROP.
PATIENTS AND METHODS
Premature infants with a birth weight of 1500 g or less (≤ 1500 g), born between 1 August 1998 and 31 July 2000 and surviving for at least 8 weeks, were included in a new, prospective population based study on the incidence of ROP in the Stockholm area of Sweden. The present geographical area was exactly the same as in the previous study1 and included four neonatal units.
Infants with a gestational age of 32 weeks or less (≤ 32 weeks) at birth, our present screening criterion,1 were also studied, but will be presented in a separate paper.
Fundus examinations were done by experienced ophthalmologists who were responsible for the various neonatal units. The aim was to perform the first eye examination 5 weeks after birth and to repeat examinations at 2 week intervals until the retina was entirely vascularised. If ROP was present, the interval was reduced to 1 week or less, depending on the stage. If examinations were stopped too early—that is, before the retina was entirely vascularised, the infant was excluded from the study. Infants were also excluded if they had their first examination too late, after completed vascularisation or later than 8 weeks after birth.
The international classification of ROP was used to describe the various stages of ROP.3,4 Each infant was classified according to the most severe stage of ROP in either of its eyes. Before examination, pupils were dilated twice with a mixture of cyclopentolate 0.5% and phenyl ephrine 0.5%. Indirect ophthalmoscopy was routinely performed. Lid speculum, scleral indentation, and topical anaesthesia were used when the border between vascularised and non-vascularised retina could not be visualised with indirect ophthalmoscopy alone. Laser treatment, as opposed to cryo treatment in the previous study,1 was applied to the avascular part of the retina anterior to the ridge. The criterion for treatment remained the same—that is, when infants had reached stage 3 ROP in at least four contiguous clock hours in zone II, even in the absence of “plus disease.” Treatment was given within 72 hours of the decision to treat.
The Swedish National Board of Health and Social Welfare provided us with data on liveborn infants with a birth weight of 1500 g or less and on the total number of liveborn infants in Stockholm county during the study period. This permitted calculation of the survival rate and dropout frequency in the study.
Statistical analysis
We used the unpaired t test to evaluate differences between the study groups (1990 and 2000) when analysing continuous data, and the Wilcoxon matched pair signed ranks test to compare the right and left eyes for the stage of ROP. To compare nominal or ordinal data, an independence test of contingency tables (χ2 test, asymptotic or exact) was performed. We analysed the effect of “study period” (1990, 2000) and “ROP grade” on birth weight and gestational age, with a two way factorial ANOVA. The interaction between “study period” and “ROP grade” was significant. Therefore simple effects tests were performed for the factor “study period” for each level of the factor “ROP grade.” Stepwise logistic regression analyses were then performed to determine the most important risk factors for ROP in the study group. The predicted probability of ROP (P) during both study periods was calculated from the logistic regression model. When comparing the years 1990 and 2000 regarding various probabilities for ROP with increasing gestational age, an interaction term between the factors “year” and “gestational age” was included in the logistic regression model. The severity of ROP was also classified into three categories—that is, no, mild, and severe. A logistic model was then performed for the study groups (1990, 2000) and the predicted probability for each category of ROP in the two study groups was calculated.
RESULTS
During the study period from 1 August 1998 to 31 July 2000, there were 38 430 liveborn infants in Stockholm county, of whom 331 (0.86 %) had a birth weight of ≤ 1500 g. Forty infants died before 8 postnatal weeks of age, leaving 291 infants with a survival rate of 87.9% at 8 weeks. Twenty four infants were never referred to us, 11 were excluded (two were examined too late and nine were completed too early), and three infants died before screening of their eyes was finished. This gave a dropout frequency of 13.1% (38/291).
The remaining 253 infants comprised the present study group and had a mean gestational age of 28.5 (range 23–34) weeks at birth, a mean birth weight of 1118 (range 462–1500)g. There were 121 girls (47.8%) and 132 (52.2%) boys; 201 infants were the products of singleton (79.4%) and 52 of multiple deliveries (20.6%).
ROP was found in 36.4% (92/253) of the infants, their mean gestational age at birth being 26.7 (23–32) weeks and their mean birth weight 929 (462–1494) g. Mild ROP (stages 1 and 2) was found in 18.2% (46/253) and severe ROP (stages 3–5) in 18.2% (46/253). We found no significant differences between the eyes and the stage of ROP.
Laser treatment was given to 31 (12.3%) infants, of whom two were operated on with a cerclage in one of their eyes.
Comparison of infants with a birth weight of ≤ 1500 g, born in 1988–1990 (“1990”) and in 1998–2000 (“2000”)
There were 260 infants in the 1990 group and 253 infants in the present 2000 group with a birth weight of ≤ 1500 g. No difference was found between the groups for sex and single versus multiple births. A slight difference was seen (p=0.02) in their mean gestational age—that is, 29.0 (24–35) weeks in the 1990 group and 28.5 (23–34) weeks in the 2000 group. Their mean birth weights were similar, 1157 (648–1500) g versus 1118 (462–1500) g.
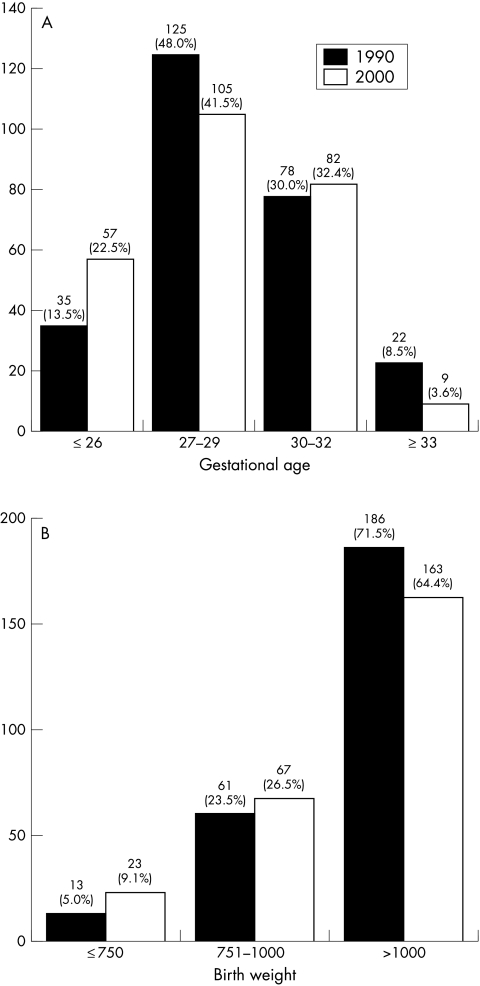
The distribution of gestational ages and birth weights in the two groups are shown in Figure 1A and B.
Figure 1.
(A) Distribution of gestational age at birth (weeks) in the two consecutive groups. (B) Distribution of birth weight (g) in the two consecutive groups.
The incidence of total, mild or severe ROP did not differ in the two groups on the whole (Table 1). When dividing infants according to gestational ages into subgroups, a significant difference in the total incidence of ROP was found in the youngest (≤ 26 weeks) group (p=0.027) and in the 30–32 week gestational age group (p=0.001) (Table 2). In the former group, the difference mainly concerned an increased frequency of severe ROP. In the latter group, there was a difference in both mild and severe ROP, a reduction in the frequency of severe ROP being most marked. Owing to the small number of infants with ROP in both groups, further analyses were unreliable.
Table 1.
Incidence of ROP in the two consecutive groups in 1990 and 2000
| No (%) patients | ||
| 1990 | 2000 | |
| Total study group | 260 | 253 |
| ROP stage 1 | 24 (9.2) | 19 (7.5) |
| ROP stage 2 | 29 (11.2) | 27 (10.7) |
| ROP stage 3 | 45 (17.3) | 44 (17.4) |
| ROP stage 4 | 6 (2.3) | 2 (0.8) |
| ROP stage 5 | 1 (0.4) | 0 (0) |
| Total ROP | 105 (40.4) | 92 (36.4) |
Table 2.
Relation of ROP to gestational age at birth in the two consecutive groups
| Gestational age at birth (weeks) | ||||||||
| ≤ 26 | 27–29 | 30–32 | ≥33 | |||||
| 1990 | 2000 | 1990 | 2000 | 1990 | 2000 | 1990 | 2000 | |
| No ROP (%) | 10 (28.6) | 6 (10.5) | 72 (57.6) | 73 (69.5) | 53 (67.9) | 73 (89.0) | 20 (90.9) | 9 (100) |
| Mild ROP (%) | 9 (25.7) | 16 (28.1) | 30 (24.0) | 23 (21.9) | 12 (15.4) | 7 (8.6) | 2 (9.1) | 0 (0) |
| Severe ROP (%) | 16 (45.7) | 35 (61.4) | 23 (18.4) | 9 (8.6) | 13 (16.7) | 2 (2.4) | 0 (0) | 0 (0) |
| Total ROP (%) | 25 (71.4) | 51 (89.5) | 53 (42.4) | 32 (30.5) | 25 (32.1) | 9 (11.0) | 2 (9.1) | 0 (0) |
| Totals | 35 | 57 | 125 | 105 | 78 | 82 | 22 | 9 |
When dividing infants into subgroups according to their birth weights (≤ 750 g, 751–1000 g, >1000 g) (Table 3), we found a significant reduction in the total incidence of ROP in the heaviest group (p=0.001). In the lightest group there were few infants and all with a birth weight ≤ 1000 g were therefore analysed together. In the latter group there was an increase in the total incidence of ROP (p = 0.039). As regards the frequencies of mild and severe ROP, the most obvious changes were an increase in severe ROP in the smallest infants and a reduction of severe ROP in the heaviest group of the “2000” infants.
Table 3.
Relation of ROP to birth weight in the two consecutive groups
| Birth weight (g) | ||||||
| ≤750 | 751–1000 | >1000 | ||||
| 1990 | 2000 | 1990 | 2000 | 1990 | 2000 | |
| No ROP (%) | 3 (23.1) | 2 (8.7) | 29 (47.5) | 23 (34.4) | 123 (66.1) | 136 (83.4) |
| Mild ROP (%) | 3 (23.1) | 4 (17.4) | 14 (23.0) | 22 (32.8) | 36 (19.4) | 20 (12.3) |
| Severe ROP (%) | 7 (53.8) | 17 (73.9) | 18 (29.5) | 22 (32.8) | 27 (14.5) | 7 (4.3) |
| Total ROP (%) | 10 (76.9) | 21 (91.3) | 32 (52.5) | 44 (65.6) | 63 (33.9) | 27 (16.6) |
| Totals | 13 | 23 | 61 | 67 | 186 | 163 |
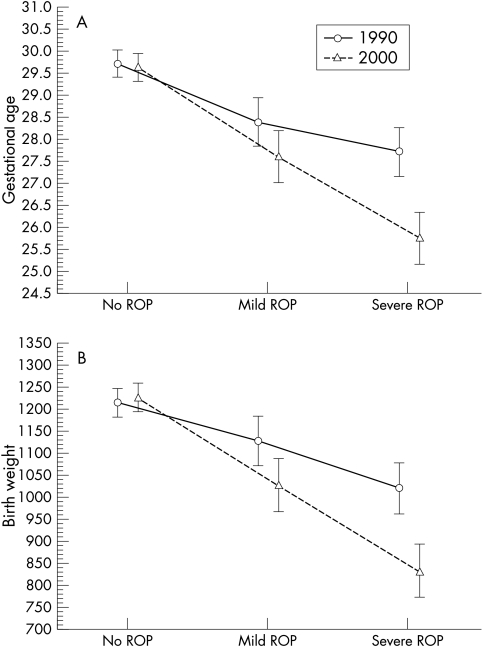
Interaction analyses to evaluate some characteristics of infants with ROP during the two periods, 1990 and 2000, were carried out. A significant interaction of mean gestational age and mean birth weight was found among the ROP infants. When further analysing mean gestational age, we found no difference in infants without ROP, a significant difference in those with mild ROP (p=0.05), and a highly significant difference in those with severe ROP (p<0.000) (Fig 2A). When analysing mean birth weight, there was no difference in infants without ROP, a significant difference in those with mild ROP (p=0.02), and a highly significant difference in those with severe ROP (p<0.000) (Fig 2B).
Figure 2.
(A) Mean gestational age at birth (weeks) and standard deviation, in relation to stage of ROP in the two consecutive groups. (B) Mean birth weight (g) and standard deviation, in relation to stage of ROP in the two consecutive groups.
Infants treated for ROP—28 cryotreated in the 1990 group and 31 laser treated in the 2000 group—also differed significantly. The mean gestational age at birth was reduced from 27.3 (24–32) weeks to 25.3 (23–28) weeks (p<0.000) and the mean birth weight from 961 (675–1380) g to 781 (462–1076) g (p<0.000).
Probability of ROP
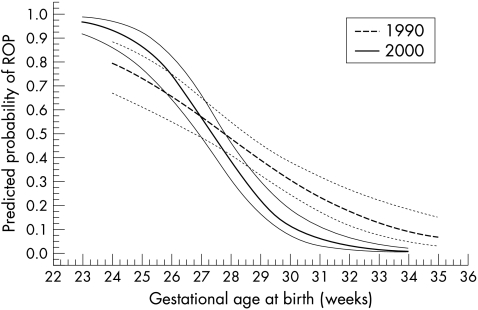
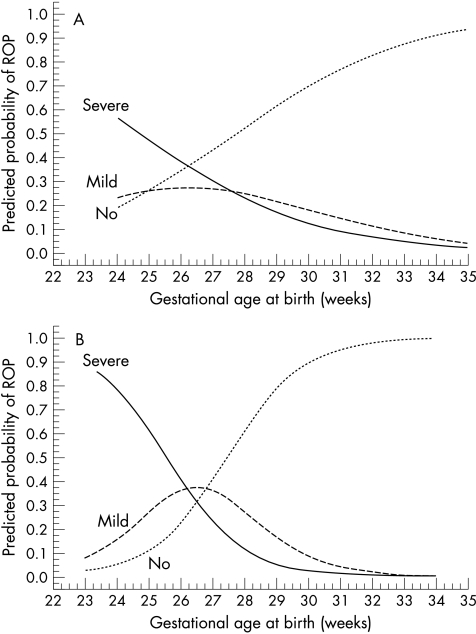
In a multiple stepwise logistic regression analysis sex, single/multiple birth, gestational age at birth, and birth weight were included as independent risk factors for ROP. Gestational age at birth was found to be the most important risk factor for ROP, followed by birth weight (Table 4) as in the previous “1990” study. When gestational age was taken into account, birth weight did not contribute further to the predicted probability of ROP, which is therefore shown only in relation to gestational age at birth (Fig 3). Interaction analysis showed a significant difference between the groups (1990 and 2000) as regards the predicted probability of ROP in relation to gestational age at birth. In the higher gestational ages, a reduced probability of ROP and in the lowest gestational ages, a greater probability of ROP in the 2000 group are seen in the figure. The confidence intervals of the low gestation infants, however, overlap and are wide in the 1990 group, making interpretations more difficult. The probabilities of no, mild, and severe ROP in the two groups are shown in Figures 4A and B.
Table 4.
Stepwise logistic regression analysis of risk factors for ROP
| Log odds ratio (b) | Standard error of b | Odds ratio | 95% CI of odds ratio | |
| Gestational age at birth | −0.5809 | 0.1183 | 1.788* | 1.418 to 2.254 |
| Birth weight | −0.00243 | 0.000916 | 1.275† | 1.065 to 1.526 |
*Odds ratio for a decrease of 1 week in gestational age.
†Odds ratio for a reduction of 100 g in birth weight.
Figure 3.
Predicted probability of ROP in relation to gestational age at birth and 95% confidence interval in the two consecutive groups (1990 and 2000) of ≤ 1500 g.
Figure 4.
(A) Predicted probability of no, mild, and severe ROP in relation to gestational age at birth in the 1990 group (≤ 1500 g). (B) Predicted probability of no, mild, and severe ROP in relation to gestational age at birth in the 2000 group (≤ 1500 g).
DISCUSSION
We repeated a population based study on the incidence of ROP in the Stockholm area of Sweden, a decade after a previous study in exactly the same geographical area.1 Inclusion criteria for the present study was a birth weight of 1500 g or less, as in the preceding study, permitting exact comparison of the incidence and severity of ROP in the population during the two periods.
The consecutive study populations (≤ 1500 g) were similar in size, although composed differently. The number of very immature (≤ 26 weeks) infants had increased in the “2000” group, which included two infants having a gestational age of 23 weeks at birth and nine of 24 weeks at birth. No infant of 23 weeks’ gestation and only four of 24 weeks’ gestation were included in the “1990” group. The survival rate at 8 weeks of infants ≤ 1500 g increased from 84% to 87.9% and of infants ≤ 1000 g from 71% to 76.5%. Improved neonatal care during the past decade, including antenatal steroids, surfactant therapy, and oscillatory ventilators, is probably the reason for this change.
The dropout groups were also similar in the two consecutive studies, being 11.9% in “1990” and 13.1 % in “2000.” However, one would have expected a lower dropout frequency today, since screening for ROP has been well established in Stockholm county during the past 10–15 years.
The incidence of total (36.4% versus 40%), mild (18.2% versus 20%), and severe ROP (18.2% versus 20%) remained the same in the “1990” and “2000” groups. However, a change occurred in the distribution of ROP. The most immature (≤ 26 weeks) and lightest (≤ 1000 g) infants seemed to have an increased risk of ROP in the “2000” group, particularly of severe ROP, while the more mature and heavy infants had a reduction in ROP, particularly of severe ROP. This finding was confirmed by analysing the predicted probability of ROP (see Figs 3 and 4A, B). It seems reasonable to believe that improved neonatal care explains the reduced risk of ROP in the more mature infants, who are probably better taken care of in the neonatal period today than they were a decade ago. Whether the increased risk of ROP in the most immature infants is reflected by an increase in the number of surviving infants in this group or if these surviving extremely immature infants also are sicker, more vulnerable, and prone to develop ROP, is debatable. Our findings, however, accord with the opinion of Vyas et al5 that advanced care with improved survival rates may increase the incidence of ROP in the most immature infants.
Gestational age at birth was the most important risk factor for ROP in both the consecutive groups of ≤ 1500 g. Although birth weight was a significant risk factor itself, it did not contribute further to the calculated probability of ROP when added to the model of the multiple stepwise logistic regression analysis. ROP is a multifactorial disease and the aetiology is not yet understood. The present study, however, is not a study of risk factors per se. Maternal and neonatal risk factors have previously been studied in this population6,7 and showed gestational age to be the major risk factor for ROP. This knowledge will be considered in future discussions on screening for ROP.
Criteria for treatment, slightly earlier than recommended by the American CRYO-ROP study,2 remained the same throughout the decade (see Methods) although the mode of treatment was changed from cryo to laser. The treated infants were also more immature and lighter in the “2000” group, in accordance with the tendency in the entire group with ROP. No treated infant had a gestational age of more than 28 weeks at birth or a birth weight above 1076 g in the “2000” group of infants ≤ 1500 g.
Evaluation of changes in the incidence of ROP requires consecutive, population based studies of exactly the same geographical areas. Only few such studies are available.8,9 In accordance with our findings, Bossi and Koerner, in a retrospective study, reported an increase in survival rate and incidence of total as well as severe ROP in infants <1000 g in Switzerland from 1983–5 to 1989–91.8 Fledelius and Dahl, however, found a reduction in total ROP in infants ≤ 1500 g (from 33% to 11%) when comparing the years 1988–93 with 1994–7 in a Danish county.9 Like us, they noticed that more and more “mature” infants escape ROP. Compared with our study they found a reduction of ROP also in those <1000 g (from 69% to 32%). Their population, however, differs from ours since the second period included no infant having ROP with a gestational age of less than 25 weeks at birth or a birth weight below 710 g, which probably accounts to some extent for the differences.
Several selected studies, both retrospective and prospective, of the incidence of ROP have been performed. Some definitely report a decrease in the incidence of total as well as severe ROP.10–12 It seems important to analyse separately the more “mature” and the more “immature” infants, to evaluate the effect of neonatal care on the incidence of ROP. In accordance with our results, many studies point to an unchanged or increased risk of ROP in the most immature infants.13–19 Although there was an unchanged incidence of ROP in the total study group, Termote et al13 and Kennedy et al,14 like us, report an increase of ROP in the most immature infants (<1000 g and <27 weeks respectively). Repka et al found a decrease in total ROP, but the incidence of threshold ROP in infants ≤ 25 weeks at birth remained the same.15 Keith and Doyle found an unchanged incidence of ROP in “mature” (≥ 1000 g) infants16 and a reduction, although not quite statistically significant, in “immature” (500–999 g) ones.17 Among the latter infants, however, those who developed severe ROP more often required treatment for the ROP, which points to a more advanced form of ROP. Todd et al reported an unchanged incidence of ROP in infants < 29 weeks of gestation during a period 1992–4 when compared to 1986–7, while a subgroup of infants with a gestational age at birth of 23–26 weeks had an increase of severe ROP during the 1990s.18 Finally, Hussein et al in a retrospective hospital based study, found a reduction in incidence and severity of ROP in their total study group, but in infants <28 weeks of gestation at birth or with a birth weight of <1000 g, there was “still a considerable risk of threshold disease.”19
Comparison of selected studies is extremely difficult, as shown above. Inclusion criteria may vary, as well as the demographics of the populations. The degree of immaturity and the survival rates are of particular interest. Conflicting results of the effect of improvements in neonatal care on the incidence of ROP have recently been commented on by Vyas et al,5 who, like us, believe there is an increase in the incidence of ROP in the most immature infants.
In conclusion, the incidence of total, mild, and severe ROP remained the same in two consecutive Swedish population based studies performed one decade after the other. The population was different in the second study group, with an increased survival of extremely immature infants and a change in the distribution of ROP. The more “mature” infants ran a lower risk of developing ROP—particularly severe ROP—while the most immature infants ran an increased risk of ROP, particularly severe ROP. We speculate that this is an effect of modern neonatology, taking better care of the more mature infants and saving the most immature and fragile infants, who previously did not survive.
Acknowledgments
We thank Elisabeth Berg, Department of Humanities, Informatics and Social Sciences, Karolinska Institute, Stockholm, who helped us with the statistical evaluation. We also thank Anna Norman, the Swedish National Board of Health and Social Welfare, for valuable help. The study was supported by Sigvard and Marianne Bernadotte Research Foundation for Children Eye Care.
REFERENCES
- 1.Holmström G, el Azazi M, Jacobson L, et al. A population-based, prospective study of the development of ROP in prematurely born children in the Stockholm area of Sweden. Br J Ophthalmol 1993;77:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol 1988;106:471–9. [DOI] [PubMed] [Google Scholar]
- 3.Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol 1984;102:1130–4. [DOI] [PubMed] [Google Scholar]
- 4.International Committee for the Classification of the Late Stages of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol 1987;105:905–12 [PubMed] [Google Scholar]
- 5.Vyas J, Field D, Draper ES, et al. Severe retinopathy of prematurity and its association with different rates of survival in infants of less than 1251 g birth weight. Arch Dis Child Fetal Neonatal Ed 2000;82:F145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmström G, Thomassen P, Broberger U. Maternal risk factors for retinopathy of prematurity – a population-based study. Acta Obstet Gynaecol Scand 1996;75:628–35. [DOI] [PubMed] [Google Scholar]
- 7.Holmström G, Broberger U, Thomassen P. Neonatal risk factors for retinopathy of prematurity—a population-based study. Acta Ophthalmol Scand 1998;76:204–7. [DOI] [PubMed] [Google Scholar]
- 8.Bossi E, Koerner F. Retinopathy of prematurity. Intensive Care M 1995;21:241–6. [DOI] [PubMed] [Google Scholar]
- 9.Fledelius H, Dahl H. Retinopathy of prematurity, a decrease in frequency and severity. Trends over 16 years in a Danish county. Acta Ophthalmol Scand 2000;78:359–61. [DOI] [PubMed] [Google Scholar]
- 10.Bullard SR, Donahue SP, Feman SS, et al. The decreasing incidence and severity of retinopathy of prematurity. J AAPOS 1999;3:46–52. [DOI] [PubMed] [Google Scholar]
- 11.Blair BM, O’Halloran HS, Pauly TH, et al. Decreased incidence of retinopathy of prematurity, 1995–1997. J AAPOS 2001;5:118–22. [DOI] [PubMed] [Google Scholar]
- 12.Rowlands E, Ionides ACW, Chinn S, et al. Reduced incidence of retinopathy of prematurity. Br J Ophthalmol 2001;85:933–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Termote J, Schalij-Delfos NE, Brouwers HAA, et al. New developments in neonatology: Less severe retinoapthy of prematurity? J Pediatr Ophthalmol Strabismus 2000;37:142–8. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy J, Todd DA, Watts J, et al. Retinopathy of prematurity in infants less than 29 weeks gestation: 3 1/2 years pre- and postsurfactant. J Pediatr Ophthalmol Strabismus 1997;34:289–92. [DOI] [PubMed] [Google Scholar]
- 15.Repka MX, Hudak ML, Parsa CF, et al. Calf lung surfactant extract prophylaxis and retinopathy of prematurity. Ophthalmology 1992;99:531–6. [DOI] [PubMed] [Google Scholar]
- 16.Keith CG, Doyle LW. Retinopathy of prematurity in infants weighing 1000–1499 g at birth. J Paediatr Child Health 1995;31:134–6. [DOI] [PubMed] [Google Scholar]
- 17.Keith CG, Doyle LW. Retinopathy of prematurity in extremely low birth weight infants. Pediatrics 1995;95:42–5. [PubMed] [Google Scholar]
- 18.Todd DA, Cassell C, Kennedy J, et al and the New South Wales neonatal intensive care units study group. Retinopathy of prematurity in infants <32 weeks’s gestation at birth in New South Wales in 1993 and 1994. J Paediatr Child Health 1999;35:355–7. [PubMed] [Google Scholar]
- 19.Hussein N, Clive J, Bhandari V. Current incidence of retinopathy of prematurity, 1989–1997. Pediatrics 1999;3:1–8. [DOI] [PubMed] [Google Scholar]