Abstract
Background: A multicentre study was carried out in Ghana and southern India to determine the aetiology of suppurative keratitis in two regions located at similar tropical latitudes. Studies of fungal keratitis from the literature were reviewed.
Methods: Patients presenting at rural and urban eye units with suspected microbial keratitis were recruited to the study. Corneal ulceration was defined as loss of corneal epithelium with clinical evidence of infection with or without hypopyon. Microscopy and culture were performed on all corneal specimens obtained.
Results: 1090 patients were recruited with suspected microbial keratitis between June 1999 and May 2001. Overall the principal causative micro-organisms in both regions were filamentous fungi (42%): Fusarium species and Aspergillus species were the commonest fungal isolates. Pseudomonas species were most frequently isolated from cases of bacterial keratitis in Ghana but in India the commonest bacterial isolates were streptococci.
Conclusion: Infections of the cornea due to filamentous fungi are a frequent cause of corneal damage in developing countries in the tropics and are difficult to treat. Microscopy is an essential tool in the diagnosis of these infections. A knowledge of the “local” aetiology within a region is of value in the management of suppurative keratitis in the event that microscopy cannot be performed.
Keywords: keratitis, Fusarium, Aspergillus
Scarring of the cornea as a result of suppurative keratitis is an important cause of preventable blindness. In some developing countries in the tropics, corneal infections are the second commonest cause of blindness after unoperated cataract.1–3 Suppurative corneal ulcers may be caused by bacteria, fungi, and protozoa. However, within the tropics, as many as two thirds of ulcers may be due to filamentous fungi. This type of ulceration is commonly associated with ocular trauma.2–9
Untreated, suppurative keratitis may lead to opacification and, ultimately, to perforation of the cornea. The associated morbidity is the result of several factors and is directly affected by difficulties in patient management because of a lack of diagnostic facilities and appropriate treatment. Specific treatment requires prompt and accurate identification of the causative micro-organisms.10 Within the setting of rural eye hospitals in the tropics laboratory facilities are rare and diagnosis is based on clinical characteristics. As a direct result of this, treatment is often empirical.
The microbial causes of suppurative keratitis vary considerably between continents and countries and also within countries. It is essential to determine the local aetiology within a given region when planning a corneal ulcer management strategy. Several studies have investigated the epidemiology of corneal ulceration, causative micro-organisms, and effective treatments, particularly in the Indian subcontinent. However there is a paucity of information in the literature with regard to the experience in African countries.6–8,11–14
The following study was conducted at hospitals in Ghana and India to compare the aetiology of suppurative keratitis in two countries, in different continents, at similar tropical latitudes. The aims of this investigation were to improve facilities for laboratory diagnosis, to determine the predominant causative micro-organisms, to identify the most suitable treatments, and encourage rapid referral of patients.
MATERIALS AND METHODS
Patients
A prospective study of suppurative keratitis was conducted in Ghana and southern India between June 1999 and May 2001. Patients were recruited at the eye unit of Korle Bu Teaching Hospital in Accra, Ghana, and also at two rural hospitals in Agogo (Ashanti region) and Bawku (Upper East region). In India, patients who presented with suppurative keratitis at two rural “taluk” hospitals in the Tiruchirapalli district of the southern state of Tamil Nadu were included in the study in addition to patients from the main study centre, Joseph Eye Hospital (JEH) base hospital.
All patients presenting with suspected suppurative keratitis were included in the study. Corneal ulceration was defined as loss of corneal epithelium with underlying stromal infiltrate and suppuration associated with signs of inflammation, with or without hypopyon.7 Patients with suspected or confirmed viral keratitis were excluded from the study. Patient consent was mandatory for inclusion in the study.
Clinical examination and laboratory investigation
Each patient was examined at the slit lamp; clinical features were noted and a drawing made for patient records. A corneal scrape was performed by an ophthalmologist using a sterile 21 gauge needle, or blunt Kimura spatula, following the instillation of local anaesthetic (amethocaine hydrochloride 0.5%, without preservative). In India, lignocaine (lidocaine) 4% was routinely administered, anaesthetic without preservative was sometimes used. If a patient was taking antibiotics at the time of presentation to the clinic treatment was stopped and investigations were delayed for 24 hours.
Corneal material obtained from scraping the ulcer was smeared onto two slides which were stained with Gram stain and lactophenol cotton blue mountant for microscopic examination. Material was inoculated directly onto 5% sheep’s blood agar, Sabouraud glucose agar, and into Sabouraud broth. In India, inhibitory mould agar (IMA) was substituted for Sabouraud glucose agar, as was routine practice of the laboratory. At the tertiary centres, if there was sufficient corneal material, additional culture media were inoculated (brain heart infusion (BHI) broth, chocolate agar, and cysteine tryptone agar). Blood agar plates, cystine tryptone agar, and BHI broth were incubated at 37°C. Sabouraud glucose agar plates, Sabouraud broth, and IMA plates were incubated at 27°C. A non-nutrient agar plate was inoculated if Acanthamoeba keratitis was suspected and an additional slide was prepared for microscopy.
Bacteria were further identified using routine biochemical identification tests and selective media. Identification of fungi was carried out in London and at the Mycology Reference Laboratory, Bristol, in addition to the overseas centres. Filamentous fungi were identified according to the macroscopic appearance of cultures on Sabouraud glucose, potato dextrose and cornmeal agars, and microscopic appearance of conidia and spore bearing structures. Yeasts were identified to species level using the germ tube test (incubation for 1.5 hours, at 37°C, in horse serum), Auxacolor and API kits (Bio Mérieux).
Microbial cultures were considered to be significant if growth of the same organism could be demonstrated on two or more solid phase cultures. Similarly, if there was semiconfluent growth at the site of inoculation or growth on one solid medium consistent with microscopy (that is, appropriate staining and morphology with Gram stain); or semiconfluent growth at the site of inoculation on one solid medium (if bacteria); or growth of the same organism on repeated scraping.1,7,15,16 If, by microscopy, hyphae were observed in corneal tissue, but failed to grow in culture, the causative organism was reported as fungal.
RESULTS
A total of 1090 patients presenting with suppurative keratitis were enrolled in the study; 290 in Ghana and 800 in India. One patient from Ghana and one patient from India presented with bilateral infection. Fungi were identified as the dominant causative agent of infection (including mixed infections): 44% in southern India and 37.6% in Ghana. Bacteria were isolated from 29.3% of cases in south India and 13.8% in Ghana (Table 1). In each case of mixed infection a single bacterial species was associated with a single fungal species. This study revealed a higher percentage of “mixed” infections in India than Ghana: 5.5% versus 1.4%.
Table 1.
Aetiology of corneal ulcers
| India | Ghana | |||
| Type of micro-organism | No | % | No | % |
| Definite bacterial | 191 | 23.9 | 36 | 12.4 |
| Definite fungal | 309 | 38.6 | 105 | 36.2 |
| Definite mixed | 44 | 5.5 | 4 | 1.4 |
| Definite Acanthamoeba spp | 7 | 0.9 | 1 | 0.3 |
| Unknown | 249 | 31.1 | 144 | 49.7 |
| Total | 800 | 100 | 290 | 100 |
| India | Ghana | |
| *Bacteria only. | ||
| Microscopy and culture negative | 23 | 87 |
| Microscopy positive, culture negative* | 218 | 24 |
| Microscopy negative, culture positive | 8 | 33 |
| Total | 249 | 144 |
Seven patients presented with suppurative keratitis due to infection with Acanthamoeba species in south India. The first reported case of Acanthamoeba keratitis in Ghana was diagnosed during this study (BJO, accepted for publication). None of these patients were contact lens wearers.
In India 249 (31%) cases had no definitive laboratory diagnosis; Gram positive cocci were reported from the microscopy in 80% (200/249) of these cases; however, cultures were negative. It was not possible to determine the aetiological agent of 144 (50%) corneal ulcer cases in Ghana (Table 1). Microscopy and culture results were negative in 60% (87/144) of cases. Scanty bacteria were seen in 14% (24) of Gram stained smears. Of the remaining 33 cases which were microscopy negative, filamentous fungi were isolated in culture from 51% cases and bacteria from 49% cases but growth was not considered to be significant, using the criteria stated (see Materials and methods).
Pseudomonas species were the commonest bacterial isolates from corneal ulcers in Ghana (52.5%); P aeruginosa being the commonest reported species. Streptococci (20%) and staphylococci (10%) were commonly associated with corneal infection (Table 2). In India streptococci accounted for 46.8% of corneal ulcers followed by staphylococci (26.8%) and pseudomonads (14.9%). In both countries more than 80% of bacterial isolates were represented by these three genera.
Table 2.
Identification of bacteria isolated from corneal tissue of patients with suppurative keratitis
| India | Ghana | |||
| Bacteria | No | % | No | % |
| Gram positive cocci | ||||
| Streptococcus spp | 110 | 46.8 | 8 | 20.0 |
| (Streptococcus pneumoniae) | (62) | (26.4) | (6) | (15.0) |
| Staphylococcus aureus | 5 | 2.1 | 2 | 5.0 |
| Coagulase –ve staphylococci | 58 | 24.7 | 2 | 5.0 |
| Micrococcus spp | – | – | 1 | 2.5 |
| Gram positive bacilli | ||||
| Bacillus spp | 2 | 0.9 | – | – |
| Corynebacterium spp | – | – | 4 | 10.0 |
| Nocardia spp | 3 | 1.3 | – | – |
| Gram negative bacilli | ||||
| Pseudomonas spp | 35 | 14.9 | 21 | 52.5 |
| (Pseudomonas aeruginosa) | (33) | (14.0) | ||
| Acinetobacter spp | 11 | 4.7 | – | – |
| Enterobacter spp | 3 | 1.3 | – | – |
| Citrobacter spp | 1 | 0.4 | – | – |
| Aeromonas spp | 5 | 2.1 | – | – |
| Klebsiella spp | 2 | 0.9 | – | – |
| Unidentified | – | – | 2 | 5.0 |
| Totals | 235 | 100 | 40 | 100 |
Fusarium spp and Aspergillus spp were isolated from 61% of all fungal infections and comprised 83% of identified fungal isolates (Table 3). Although Fusarium species were the most prevalent fungal pathogens reported in Ghana, differences in the spectra of fungi were observed between the participating centres. In Accra (southern Ghana), 63% (27/43) of fungi isolated were Fusarium spp compared with 31.3% (15/48) at Bawku Hospital (northern Ghana), where a greater variety of fungi were isolated. Lasiodiplodia theobromae was the third commonest fungal isolate in Ghana. In India a greater number, and diversity, of dematiaceous moulds were cultured; Curvularia species were most frequently isolated.
Table 3.
Identification of fungi isolated from corneal tissue of patients with suppurative keratitis
| India | Ghana | |||
| Fungi | No | % | No | % |
| Fusarium spp | 141 | 39.9 | 46 | 42.2 |
| Aspergillus spp | 76 | 21.5 | 19 | 17.4 |
| A flavus | 59 | (16.7) | 9 | (8.3) |
| A fumigatus | 15 | (4.2) | 7 | (6.4) |
| A niger | 1 | (0.3) | 1 | (0.9) |
| A nidulans | – | – | 1 | (0.9) |
| Aspergillus spp | 1 | (0.3) | 1 | (0.9) |
| Cuvularia spp | 34 | 9.6 | 1 | 0.9 |
| Lasiodiplodia theobromae | 1 | 0.3 | 6 | 5.5 |
| Paecilomyces spp | – | – | 1 | 0.9 |
| Penicillium spp | 2 | 0.6 | – | – |
| Scedosporium apiospermum | 2 | 0.6 | 2 | 1.8 |
| Cephaliophora irregularis | 1 | 0.3 | – | – |
| Cladosporium cladosporoides | – | – | 1 | 0.9 |
| Cylindrocarpon spp | 1 | 0.3 | – | – |
| Exserohilum rostratum | 1 | 0.3 | – | – |
| Bipolaris spp | 2 | 0.6 | – | – |
| Candida spp | – | – | 1 | 0.9 |
| Unidentified | 35 | 9.9 | 5 | 4.6 |
| Suspected, but culture negative | 57 | 16.1 | 27 | 24.8 |
| Totals | 353 | 100 | 109 | 100 |
DISCUSSION
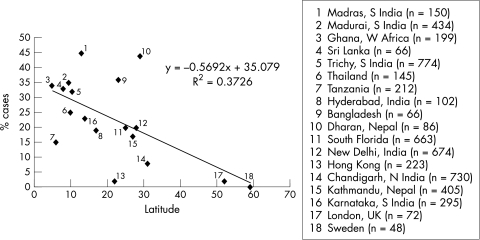
Fungi were identified as the principal aetiological agent of corneal ulceration: 44% and 37.6% of cases in India and Ghana. Earlier studies in the same regions reported a similarly high proportion of fungal keratitis.6,12 A review of the literature shows that there are distinct patterns of geographical variation in the aetiology of suppurative keratitis and considerable variation in the proportion due to fungi has been documented (Table 4). The proportion of corneal ulcers caused by filamentous fungi increases towards tropical latitudes (Fig 1). In more temperate climates, fungal ulcers are uncommon and are more frequently associated with Candida species than filamentous fungi.21–24 Houang et al43 reviewed the relation of fungal keratitis to climate concluding that, although a higher incidence of fungal keratitis could be expected in countries with similar annual rainfall and temperature range, this was not always so and was also dependent on the extent of urbanisation.
Table 4.
Mycotic keratitis: a review of the literature
| Place | Reference | Year | Number cases | % Fungi* | Organism 1 | Organism 2 |
| Europe | ||||||
| London | Coster17 | 1981 | 67 | 3% | 2 cases only | |
| London, UK | Personal comm | 2001 | 72 | 3% | Candida spp 58% | Aspergillus spp 26% |
| Sweden | Neumann18 | 1993 | 48 | 0% | ||
| North America | ||||||
| Florida | Liesegang19† | 1980 | 663 | 20% | Fusarium spp 62% | Candida spp 7.5% |
| Florida | Rosa20 | 1994 | 125 | Fusarium spp 62% | Candida spp 12.5% | |
| Philadelphia | Tanure 21 | 2000 | 24 | C albicans 46% | Fusarium spp 25% | |
| California | Ormerod22† | 1987 | 227 | 11% | C albicans 4% | Penicillium spp 2% |
| Atlanta | Harris23 | 1988 | 108 | 32% | Candida spp 94% | |
| Northern USA | O’Day24 | 1987 | 33 | Candida spp 42% | Aspergillus spp 30% | |
| Southern USA | 285 | Fusarium spp 55% | Candida spp 9.5% | |||
| South America | ||||||
| Paraguay | Mino de Kaspar25 | 1991 | 26 | 58% | Fusarium spp 42% | Aspergillus spp 19% |
| The Middle East | ||||||
| Saudi Arabia | Khairallah26 | 1992 | 191 | 14% | Aspergillus spp 41% | Fusarium spp‡ |
| Candida albicans‡ | ||||||
| Africa | ||||||
| Ghana (Accra) | Hagan6† | 1995 | 199 | 34% | Fusarium spp 52% | Aspergillus spp 15% |
| South Africa | Carmichael27 | 1985 | 274 | 2% | Curvularia spp 33% | |
| Nigeria | Gugani11 | 1976 | 59 | Fusarium spp 36% | Penicillium spp 29% | |
| Tanzania | Mselle28 | 1999 | 212 | 15% | Fusarium spp 75% | Aspergillus spp 19% |
| South Africa | Ormerod29 | 1987 | 120 | 2.5% | 3 different isolates | |
| Asia | ||||||
| Nepal, Dharan | Khanal9† | 2001 | 86 | 44% | Aspergillus spp 60% | Fusarium spp 13% |
| Nepal, Kathmandu | Upadhyay1 | 1991 | 405 | 17% | Aspergillus spp 47% | Candida spp 13% |
| Nepal | Chaudhary30 | 1999 | 110 | 8% | Rhizopus spp 22% | |
| Bangladesh | Rahman31 | 1998 | 63 | Aspergillus spp 35% | ||
| Fusarium spp 35% | ||||||
| Bangladesh | Dunlop4 | 1994 | 66 | 36% | Aspergillus spp 40% | Fusarium spp 21% |
| Bangladesh | Williams32† | 1991 | 127 | 34% | Aspergillus spp 49% | Fusarium spp 28% |
| Bangladesh | Williams33† | 1987 | 33 | 21% | Aspergillus spp 29% | Fusarium spp 14% |
| India, North | Chander34 | 1994 | 730 | 8.4% | Aspergillus spp 40% | Fusarium spp 16% |
| India, New Delhi | Panda (children)8 | 1997 | 211 | 10.8% | Aspergillus spp 40% | Fusarium spp 11% |
| India, New Delhi | Mahajan35 | 1985 | 674 | 19.7% | Aspergillus spp 37% | Fusarium spp 10% |
| India, Mumbai | Deshpande13† | 1999 | 367 | Aspergillus spp 60% | Candida spp 10% | |
| India, Hyderabad | Kunimoto16† | 2000 | 102 | 19% | Aspergillus spp 37% | Curvularia spp 16% |
| India, Hyderabad | Garg14 | 2000 | 557 | Fusarium spp 38% | Aspergillus spp 30% | |
| India, Karnataka | Kotigadde36 | 1992 | 295 | 23% | Aspergillus spp 34% | Candida spp 19% |
| India, Madras | Sudaram37 | 1989 | 150 | 45% | Aspergillus spp 53% | Fusarium spp 12% |
| Penicillium spp 12% | ||||||
| India, Madras | Venugopal38† | 1989 | 322 | Aspergillus spp 64% | Acremonium spp 8.4% | |
| India, Tiruchiripalli | Thomas12 | 1986 | 774 | 32% | Fusarium spp 38% | Aspergillus spp 30% |
| India, Madurai | Rahman39 | 1997 | 58 | Fusarium spp 38% | Aspergillus spp 17% | |
| India, Madurai | Srinavasan7† | 1997 | 434 | 35% | Fusarium spp 47% | Aspergillus spp 16% |
| Sri Lanka | Gonawardena40 | 1994 | 66 | 33% | Aspergillus spp 18% | single isolates |
| Thailand | Imwidthaya41 | 1995 | 145 | 25%‡ | Aspergillus spp 34% | Fusarium spp 26% |
| Singapore | Wong42 | 1997 | 29 | Fusarium spp 52% | A flavus 17% | |
| Hong Kong | Houang43 | 2001 | 223 | 2% | Fusarium spp 60% |
*Corresponds to the proportion of fungal keratitis cases in studies which were not exclusively reporting fungal keratitis.
†Percentages of fungal keratitis cases have been recalculated by the author to represent the proportion of fungal corneal ulcers as a percentage of the total number of corneal ulcers reported, not just those which were culture positive. Numbers of cultures can be misleading particularly if multiple isolates are cultured from the same patient.
‡No figures stated.
Figure 1.
Suppurative keratitis due to fungi as a proportion of total number of cases, by latitude.
Aspergillus spp and Fusarium spp are the most frequently reported fungal pathogens isolated from cases of fungal keratitis in the tropics.5 In both Ghana and south India the most commonly isolated fungal pathogens in the current series were Fusarium spp. Other studies in south India have reported Fusarium spp to be more common than Aspergillus species. Fusarium spp have also been found to be the principal fungal pathogen in Florida, Paraguay, Nigeria, Tanzania, Hong Kong, and Singapore (Table 4). Aspergillus species predominate in northern India, Nepal, and Bangladesh.1,9,13,32,34 This phenomenon may be explained by differences in climate and the natural environment. A similar pattern was also observed in Ghana. Although Fusarium spp were the most commonly isolated fungi at all of the centres in Ghana, moulds with enteroblastic conidia adhering in dry chains—for example Aspergillus spp and Paecilomyces spp, were more frequently isolated from patients in the north of the country where the environment is drier and dustier, than in the more humid south. As observed by Khairallah26 the high proportion of corneal infections caused by Aspergillus spp in drier climes may be due to the fact that spores of Aspergillus spp can tolerate hot, dry weather conditions. Aspergillus spp also predominate in more temperate latitudes.
A significant increase in the number of reported cases of suppurative keratitis was observed during the harvest period and windy seasons. However, the proportion of corneal ulcers due to fungi remained consistently high throughout the year. Other authors have made similar observations, noting an increase in cases of fungal keratitis during the dry, windy seasons compared with the wet, humid months of the year.4,19 This trend is likely to be a direct consequence of increased agricultural activity before and immediately following the rains. Some studies have reported an increase during the hot and humid months.8,35
The majority of filamentous fungi associated with corneal ulceration in the tropics are saprobic, thermophilic moulds that are found widely in this environment. These are ubiquitous in the soil and vegetation. Fusarium species are common plant pathogens, particularly of cereal crops or saprophytes of plant debris and are found in soil.44 The aspergilli are ubiquitous and have been found almost everywhere on every conceivable type of substrate, including soil and decaying organic debris. Some of the less common isolates such as Bipolaris spp and Exserohilum spp are pathogens of grasses. Curvularia spp mostly occur on dead plant material.44 Although injury by vegetable matter is considered to be predictive for fungal keratitis, in this study fungal corneal ulcers were more often preceded by dust or mud particles in the eye.
A shift in the predominant bacterial pathogens was observed when compared with earlier findings. In Ghana, >50% of bacterial isolates were Pseudomonas species compared with 27% of isolates in a previous study by Hagan et al.6 There is a paucity of information in the literature regarding the aetiology of bacterial corneal ulcers in sub-Saharan tropical Africa and, therefore, comparisons are only possible with similar geographical and climatic regions outside the continent of Africa. Pseudomonas species were identified as the commonest bacterial isolate in a study of 142 cases of suppurative keratitis in Bangladesh in a study by Dunlop et al and also by Williams et al, who found that 40% of bacterial isolates were Pseudomonas spp.4,33 A predominance of Pseudomonas species has been reported in Hong Kong, Florida, and Paraguay.19,25,43 In reports from Nepal and south India, Gram positive cocci have been reported as the primary cause of bacterial keratitis.1,7,9 In this study, Gram positive cocci accounted for the majority (74%) of bacterial isolates in India, as found previously by Thomas et al.12 The proportion of bacterial ulcers caused by Streptococcus spp increased from 18.5% (1986) to 46.8% in this study. Similar diagnostic criteria were used in the previous study and, therefore, the trend may be attributable to a genuine change of bacterial flora within the geographical area, as influenced by climate and environment.
Traditional diagnostic laboratory methods, including microscopy and culture may be negative despite a clear clinical presentation of suppurative keratitis. This was true in 50% and 31% of cases in Ghana and India, respectively. This may be due to difficulty in obtaining sufficient corneal material for conventional investigation. This applies particularly to large, late stage ulcers, because of the risk of perforation and, conversely, early stage, small ulcers from which little material is available. It is imperative that the quality and quantity of specimen is optimal for accurate laboratory diagnosis. Self administration of antibiotics by patients before seeking medical attention has been thought to affect the recovery of organisms in culture.4 It is possible culture positivity correlated with inappropriate antibiotic usage.
Although there were a high proportion of corneal ulcers in Ghana for which the aetiology was not determined, the number of proved fungal ulcers at each of the centres was greater than the number of proved bacterial ulcers. It is therefore not unreasonable to assume that this trend could be extrapolated.
It is usually not possible to determine the significance of bacteria observed by microscopy alone. Small numbers of Gram positive cocci may be contaminants from the lid margin. Conversely, the presence of fungal hyphae in corneal tissue is significant. In agreement with Dunlop et al the sensitivity, specificity, and predictive value of Gram stain microscopy is much higher for fungal ulcers than those caused by bacteria.4 In this study 95% of fungal infections could have been diagnosed based on the findings from microscopy alone. This is an important conclusion, since the majority of rural based clinics in areas where suppurative keratitis is a problem do not have culture facilities but may be able to perform simple microscopy. A wet mount preparation, using lactophenol cotton blue stain, was used as a supplementary stain in this study. Where scientific expertise, and/or resources, may be an issue, it is easier to observe fungal hyphae employing this method than using the potassium hydroxide (KOH) technique. Reports into the presence of fungi in the eyes of asymptomatic individuals, have shown that a wide variety of fungi may be transient in the conjunctival sac. This may be the case in as many as 37% of healthy eyes, thereby discounting the use of single culture.11
In conclusion, it is imperative to know the “local” aetiology of keratitis in a particular region. Our comparative study demonstrates that there were differences between two countries with similar tropical climates at the same latitude. Equally, differences were observed within the same country. This is important information with regard to management, as many eye units do not have microscopy or culture facilities. Diagnosis is then dependent on clinical acumen and the treatment provided is, at best, empirical. It is clear that it is important to know the local aetiology, particularly if diagnosis is going to be reliant on clinical signs alone. Changing patterns of disease have also occurred in temperate, developed countries, as exemplified by the increase in keratitis due to Pseudomonas spp and Acanthamoeba spp in recent years. Awareness of changes in aetiology and antimicrobial resistance, when this information is available, are critical to managing keratitis cases.
Acknowledgments
The authors would like to thank the staff of Korle Bu, Bawku, and Agogo hospitals (Ghana) and Musiri, Ariyalur, and Myladthurai hospitals (India) for their assistance during this study. Also, Miss Linda Ficker, Dr Allen Foster, and Dr Elizabeth Wright for their clinical guidance.
REFERENCES
- 1.Upadhyay MP, Karmacharya PC, Koirala S, et al. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am J Ophthalmol 1991;111:92–9. [DOI] [PubMed] [Google Scholar]
- 2.Gonzales CA, Srinivasan M, Whitcher JP, et al. Incidence of corneal ulceration in Madurai District, south India. Ophthalmic Epidemiol 1996;3:159–66. [DOI] [PubMed] [Google Scholar]
- 3.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ 2001;79:214–21. [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlop AAS, Wright ED, Howlader SA, et al. Suppurative corneal ulceration in Bangladesh. Aus NZ J Ophthalmol 1994;22:105–10. [DOI] [PubMed] [Google Scholar]
- 5.Thomas PA. Mycotic keratitis—an underestimated mycosis. J Med Vet Mycol 1994;32:235–56. [DOI] [PubMed] [Google Scholar]
- 6.Hagan M, Wright E, Newman M, et al. Causes of suppurative keratitis in Ghana. Br J Ophthalmol 1995;79:1024–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and etiological diagnoses of corneal ulceration in Madurai, south India. Br J Ophthalmol 1997;81:965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panda A, Sharma N, Das G, et al. Mycotic keratitis in children: epidemiologic and microbiologic evaluation. Cornea 1997;16:295–9. [PubMed] [Google Scholar]
- 9.Khanal B, Kaini KR, Deb M et al. Microbial keratitis in eastern Nepal. Tropical Doctor 2001;31:168–9. [DOI] [PubMed] [Google Scholar]
- 10.Foster CS. Fungal keratitis. Infect Dis Clin N Am 1992;6:851–7. [PubMed] [Google Scholar]
- 11.Gugani HC, Talwar RS, Njoku-Obi AN, et al. Mycotic keratitis in Nigeria. A study of 21 cases. Br J Ophthalmol 1976;60:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas PA, Kalavathy CM, Rajasekaran J. Microbial keratitis—a study of 774 cases and review of the literature. J Madras State Ophthalmic Assoc 1986;23:13–21. [Google Scholar]
- 13.Deshpande SD, Koppikar GV. A study of mycotic keratitis in Mumbai. Indian J Pathol Microbiol 1999;42:81–7. [PubMed] [Google Scholar]
- 14.Garg P, Gopinathan U, Choudhary K, et al. Keratomycosis: clinical and microbiologic experience with dematiaceous fungi. Ophthalmology 2000. 107:574–80. [DOI] [PubMed] [Google Scholar]
- 15.Jones DB. Initial therapy of suspected microbial corneal ulcers. II. Specific antibiotic therapy based on corneal smears. Surv Ophthalmol 1979;24:97, 105–61. [DOI] [PubMed] [Google Scholar]
- 16.Kunimoto DY, Sharma S, Garg P, et al. Corneal ulceration in the elderly in Hyderabad, south India. Br J Ophthalmol 2000;84:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coster DJ, Wilhelmus J, Peacock J, et al. Suppurative keratitis in London. The cornea in health and disease. VIth Congress of the European Society of Ophthalmology 1981;40:395–8. [Google Scholar]
- 18.Neumann M, Sjostrand J. Central microbial keratitis in a Swedish city population. A three-year prospective study in Gothenburg. Acta Ophthalmol (Copenh) 1993;71:160–4. [DOI] [PubMed] [Google Scholar]
- 19.Liesegang TJ, Forster RK. Spectrum of microbial keratitis in south Florida. Am J Ophthalmol 1980;90:38–47. [DOI] [PubMed] [Google Scholar]
- 20.Rosa RH, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in south Florida. Ophthalmology 1994;101:1005–13. [DOI] [PubMed] [Google Scholar]
- 21.Tanure MA, Cohen EJ, Sudesh S, et al. Spectrum of fungal keratitis at Wills Eye Hospital Philadelphia, Pennsylvania. Cornea 2000;19:307–12. [DOI] [PubMed] [Google Scholar]
- 22.Ormerod LD, Hertzmark E, Gomez DS, et al. Epidemiology of microbial keratitis in southern California. A multivariate analysis. Ophthalmology 1987;94:1322–33. [DOI] [PubMed] [Google Scholar]
- 23.Harris DJ Jr, Stulting RD, Waring GO 3rd, et al. Late bacterial and fungal keratitis after corneal transplantation. Spectrum of pathogens, graft survival, and visual prognosis. Ophthalmology 1988;95:1450–7. [DOI] [PubMed] [Google Scholar]
- 24.O’Day DM. Selection of appropriate antifungal therapy. Cornea 1987;6:238–45. [DOI] [PubMed] [Google Scholar]
- 25.Miño de Kaspar H, Zoulek G, Paredes ME, et al. Mycotic keratitis in Paraguay. Mycoses 1991;34:251–4. [DOI] [PubMed] [Google Scholar]
- 26.Khairallah SH, Byrne KA, Tabbara KF. Fungal keratitis in Saudi Arabia. Doc Ophthalmol 1992;79:269–76. [DOI] [PubMed] [Google Scholar]
- 27.Carmichael TR, Wolpert M, Koornhof HJ. Corneal ulceration at an urban African hospital. Br J Ophthalmol 1985;69:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mselle J. Fungal keratitis as an indicator of HIV infection in Africa. Trop Doct 1999;29:133–5. [DOI] [PubMed] [Google Scholar]
- 29.Ormerod D. Causation and management of microbial keratitis in subtropical Africa. Ophthalmology 1987;94:1662–8. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhary A, Singh TSK, Lalchandani S, et al. Corneal ulceration and microbial keratitis in Pokhara, Nepal. J Nep Med Assoc 1999;39:18–22. [Google Scholar]
- 31.Rahman MR, Johnson GJ, Husain R, et al. Randomised trial of 0.2% chlorhexidine gluconate and 2.5% natamycin for fungal keratitis in Bangladesh. Br J Ophthalmol 1998;82:919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams G, McClellan K, Billson F. Suppurative keratitis in rural Bangladesh: the value of gram stain in planning management. Int Ophthalmol 1991;15:131–5. [DOI] [PubMed] [Google Scholar]
- 33.Williams G, Billson F, Husain R, et al. Microbiological diagnosis of suppurative keratitis in Bangladesh. Br J Ophthalmol 1987;71:315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chander J, Sharma A. Prevalence of fungal corneal ulcers in northern India. Infection 1994;22:207–9. [DOI] [PubMed] [Google Scholar]
- 35.Mahajan VM. Ulcerative keratitis: an analysis of laboratory data in 674 cases. J Ocul Ther Surg 1985;4:138–41. [Google Scholar]
- 36.Kotigadde S, Ballal M, Jyothirlatha, et al. Mycotic keratitis: a study in coastal Karnataka. Indian J Ophthalmol 1992;40:31–3. [PubMed] [Google Scholar]
- 37.Sundaram BM, Badrinath S, Subramanian S. Studies on mycotic keratitis. Mycoses 1989;32:568–72. [DOI] [PubMed] [Google Scholar]
- 38.Venugopal PL, Venugopal TL, Gomathi A, et al. Mycotic keratitis in Madras. Indian J Pathol Microbiol 1989;32:190–7. [PubMed] [Google Scholar]
- 39.Rahman MR, Minassian DC, Srinivasan M, et al. Trial of chlorhexidine gluconate for fungal corneal ulcers. Ophthalmic Epidemiol 1997;4:141–9. [DOI] [PubMed] [Google Scholar]
- 40.Gonawardena SA, Ranasinghe KP, Arseculeratne SN, et al. Survey of mycotic and bacterial keratitis in Sri Lanka. Mycopathologia 1994;127:77–81. [DOI] [PubMed] [Google Scholar]
- 41.Imwidthaya P. Mycotic keratitis in Thailand. J Med Vet Mycol 1995;33:81–2. [DOI] [PubMed] [Google Scholar]
- 42.Wong TY, Fong KS, Tan DTH. Clinical and microbial spectrum of fungal keratitis in Singapore: a 5-year retrospective study. Int Ophthalmol 1997;21:127–30. [DOI] [PubMed] [Google Scholar]
- 43.Houang E, Lam D, Fan D, et al. Microbial keratitis in Hong Kong: relationship to climate, environment and contact-lens disinfection. Trans R Soc Trop Med Hyg 2001;95:361–7. [DOI] [PubMed] [Google Scholar]
- 44.De Hoog GS, Guarro J, Gené J, et al. Atlas of clinical fungi. 2nd ed. 2000:30–5.