Abstract
Background/aims: Antimicrobial activity in tears prevents infection while maintaining a commensal bacterial population. The relation between mucin and commensal bacteria was assessed to determine whether commensals possess mucinolytic activity, how degradation depends on mucin integrity, and whether mucins affect bacterial replication.
Methods: Bacteria were sampled from healthy eyes and contact lenses from asymptomatic wearers. Intracellular mucins were extracted and purified from cadaver conjunctivas, and surface mucins from extended wear contact lenses. After exposure to bacteria, changes in mucin hydrodynamic volume (proteolytic cleavage) and subunit charge (oligosaccharide degradation) were assayed by size exclusion and ion exchange chromatography. The effect of mucin on bacterial replication was followed for up to 24 hours from the end of incubation with purified ocular mucins.
Results: Ocular bacteria decreased the hydrodynamic volume of intracellular and contact lens adherent mucins, irrespective of glycosylation density. A decrease in mucin sialylation was observed after exposure to commensal bacteria. Subunit charge distributions were generally shifted to lesser negative charge, consistent with loss of charged epitopes. Subunits with high negative charge, observed after digesting lightly adhering contact lens mucins with bacteria, suggest preferential cleavage sites in the mucin molecule. The presence of purified ocular mucin in the medium inhibited bacterial growth.
Conclusion: Bacteria in the healthy ocular surface possess mucinolytic activity on both intact and surface processed mucins, targeted to discrete sites in the mucin molecule. Inhibition of bacterial growth by ocular mucins can be seen as part of the mucosal control of microbiota.
Keywords: bacteria, contact lens, mucin glycoprotein
Mucin production is an evolutionary defence mechanism. It is increasingly evident that mucins are far from passive entities in interactions with bacteria. On the one hand bacterial colonisation of a mucosa induces far reaching changes in the physiology of the host,1 on the other hand mucin secretion together with the secretion of other antibacterials2 are expected to diminish bacterial colonisation. Antibacterials in tears—for example, lysozyme, betalysin, lactoferrin, lipoglycans, sIgA,3 and defensins,4–6 presumably together with preocular mucins, maintain bacteria at such low levels that 40% of ocular surfaces are culture negative.3
Adhesion of bacteria to mucins from other systems involves both carbohydrate moieties and bacterial protein receptors.7–12 Mucin sulphation, sialylation, and the pH of the gel have been shown to modulate these interactions.12–16
Mucin has been shown to decrease bacterial adherence to the cornea.17 However, despite the presence of mucin in the preocular fluid18 and on contact lenses,19 the number of colony forming units and the diversity of bacterial species is increased in asymptomatic contact lens wear.20,21 Factors which might contribute to increased bacterial diversity and numbers include acidification22 and changes in the osmolarity of tears under a contact lens, that, in turn, may engender changes in mucin structure and influence bacterial adherence.23 Slower mucin turnover might also improve the probability of sampling bacteria from the ocular surface.
We cultured aerobic bacteria from healthy ocular surfaces of non-contact lens and contact lens wearers. Bacterial effects on the biochemical characteristics of purified mucins were evaluated to assess the mucinolytic, proteolytic, and glycolytic activity. Two types of purified human ocular mucin were tested: intracellular mucins, extracted from conjunctival cells and mucins that had adhered to extended wear contact lenses. The first represent mucins that had not been altered by interactions with tear film components. The latter were trapped in the tear film by their adherence to contact lenses, and therefore more likely to have been maximally acted upon by tear film agents. Mucin purification eliminates contamination from other antimicrobial activities, thus isolating those activities related to mucins.
METHODS
Sampling and culture of bacteria
The use of volunteers followed the tenets of the Declaration of Helsinki and received ethical approval from the local research ethics committee.
Bacteria from the lower bulbar conjunctiva of 21 healthy individuals who did not, or only very occasionally, wore contact lenses were sampled with sterile impression cytology filters (Millipore, Bedford, MA, USA). Bacteria were grown on agar substrates to obtain commensal microbiota samples and to assess whether this method is comparable to more standard sampling techniques.
Bacteria were cultured from worn contact lenses to increase the spectrum of non-pathogenic bacteria whose mucinolytic activity was assessed. All contact lenses used in this study had been discarded in the clinic because of spoilage as a result of tear deposits. They had been worn continuously by 75 asymptomatic patients (age 37–96, median 82; mode 89; 26 M/49 F) attending the optometry department at the Bristol Eye Hospital for their contact lens management. Lens types included 56% I78 (Copolymer Filcon 3a, 79% water content, Dk × 10−11 = 64); 19% E74 (Filcon 4a Polyxylon, 74% water content, Dk × 10−11 = 33); and 15% PCM (Filcon 4a Polyxylon, 79% water content, Dk × 10−11 = 57). The duration of continuous wear was similar in men and women, with means of 63 and 65 days, respectively, and medians 55 and 61 days.
Lenses were placed in sterile saline until processed at the end of the clinic. The lenses were bisected with a sterile scalpel; half was exposed for 5 minutes to 150 μl preservative free N-acetyl cysteine 5%, (NAcCys, Moorfields Hospital, London, UK), the other half was scraped with blunt tweezers in 300 μl tryptic soy broth (TSB, Sigma, Poole, UK), 20 sweeps on each side. A volume of 150 μl of supernatant was inoculated on blood agar. Hemi-lenses were impressed onto the same medium. All cultures were kept at 35°C with 5% carbon dioxide and inspected after 1, 3, and 7 days when colonies were counted. Some isolates were subcultured in TSB and kept, cryoprotected, at −18°C.
Mucins and mucinolytic activity
Intracellular mucins were extracted from fragments of cadaver conjunctiva. Surface mucins were obtained from spoilt contact lenses worn by asymptomatic patients. Extraction was performed in 4M guanidinium chloride (GuHCl, Sigma, purified on active charcoal) with protease inhibitors.24 Contact lens adherent mucins were extracted in GuHCl as above, followed by two further extractions in fresh portions of GuHCl containing 10 mM dithiothreitol (DTT, Sigma). DTT cleaves disulphide bonds and thus dissolves aggregates25 containing both polymeric mucins (which contain disulphide links) and membrane mucins non-covalently linked to the former.
Mucins were isolated on 4M GuHCl-caesium chloride (CsCl, Sigma) gradients to ensure separation from proteins and peptides (that band at the top of the gradient) and nucleic acids (banding at the bottom).24,26 Further purification was achieved by size exclusion chromatography on Sepharose CL2B (Amersham Pharmacia Biotech, Uppsala, Sweden). The largest (Vo) intracellular mucins within narrow buoyant density ranges (that is, 1.25–1.3 g/ml; 1.3–1.35, 1.35–1.4, and 1.4–1.5 g/ml) were used in this study. These fractions contain the purest mucins, and their large size optimises detection of changes in hydrodynamic volume. Similar densities of glycosylation (that dictate banding density) were used to assess whether bacteria can degrade mucin oligosaccharides and whether their effect is related to the frequency of oligosaccharide chains on the mucin molecule. Before incubation with bacteria mucins were dialysed against three changes of sterile PBS to eliminate any salts that might interfere with bacterial growth or enzyme activity.
To establish whether incubation with mucins modifies bacterial growth, volumes of 150 μl mucins each were mixed with 50 μl bacteria (11–13 isolates from the ocular surface and 14–16 from contact lenses) and incubated for 5 minutes, 30 minutes, 1 hour, 4 hours, and 8 hours after which 10 μl of the mixture were diluted in 200 μl TSB. Bacterial proliferation, assessed as optical density at 450 nm, was evaluated for up to 24 hours after the end of exposure to mucins. All incubations were performed at 35°C and in 5% carbon dioxide.
To assess mucinolytic activities, the largest mature human ocular mucins, suspended in phosphate buffer saline, were incubated for 16 hours with bacterial isolates from the ocular surface (3A, 13B, 21C) and contact lenses (P30, R30, R37). Excess 4M GuHCl with protease inhibitors24 was added at the end of this period to halt bacterial action. Changes in molecular hydrodynamic volume were followed by size exclusion chromatography in 4M GuHCl on Sepharose CL2B. Agarose and polyacrylamide gel electrophoresis27 were used to refine degradation profiles. After reduction and alkylation, distributions of subunit charge were assessed by ion exchange chromatography on MonoQ (Pharmacia).28 Alkylation prevents the reformation of disulphide bonds.
Presence of MUC5AC, a major ocular surface mucin, was assessed by immunoreactivity with antibody antiM129 (gift from Jaques Bara, Paris, France) to epitopes in the C-terminal region of the peptide core. The oligosaccharide epitope sialylTn, that is involved in mucin bacteria interactions12,15 and widely distributed on ocular mucins,24 was visualised by reaction with antibody TKH2.30 These reactivities were followed in eluents dot blotted on Immobilon (Immobilon P, Millipore, Bedford, MA, USA). The presence of mucins on vacuum or electroblots was tested by incubation with horse radish peroxidase (HRP) tagged wheat germ agglutinin (WGA, Sigma).27,28
RESULTS
Bacterial yields
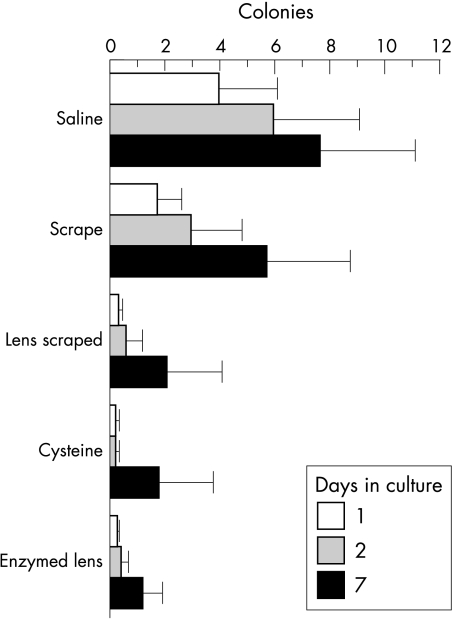
Of the 21 impression cytology samples from volunteers, 25% were positive on day 2, with six or fewer colonies in all but one case. Half of the 75 contact lens supernatants were culture positive 24 hours after plating, three quarters after 1 week. A similar trend was observed for lens deposits dislodged by scraping: 48% were culture positive 24 hours after plating and 67% after 1 week. Treatment with N-acetyl cysteine, which dissolves aggregates by breaking disulphide bonds, released bacteria which grew after 1 day in 28% of lenses and after 1 week in 56% of cases. Half the scraped or NAcCys treated contact lenses directly impressed on agar were culture negative after 1 week. In 19 cultures, too many colonies to be counted were obtained from at least one method of sampling during 1 week of culture. Most of the positive cultures, however, yielded a small number of colonies (Fig 1). After 1 day in culture, 1–10 colonies were observed in 34% of lens supernatants, 26% scraped lens deposits, and 23% of enzymatically removed deposits. After 7 days 23% of supernatants, 32% of scraped lens deposits, and 32% of enzymatically removed deposits still contained between one and 10 colonies. An almost equal number of colonies were obtained from the saline supernatant and scraping, fewer after enzymatic treatment (Fig 1). A control experiment established that bacterial growth was not affected by N-acetyl cysteine. There was no correlation between the number of colonies obtained by the different methods of sampling.
Figure 1.
Bacterial yields from lenses. The mean number of colonies (95% confidence interval) is shown for each sampling method after 1, 2, or 7 days in culture. Too many colonies to be counted (over 50) were obtained from at least one sampling mode in 26% of lenses. These are omitted from the graph. Taking into account all lenses, lens material did not have a significant effect on bacterial load (Manova, p = 0.31).
Bacterial replication in the presence of mucins
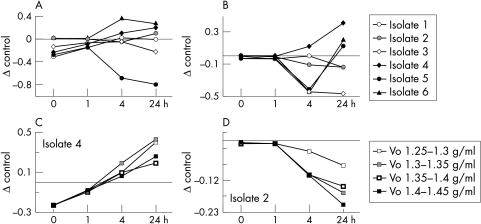
At the end of the exposure period, the density of bacteria incubated with mucins was similar to that of control cultures maintained in TSB, irrespective of mucin buoyant density (Fig 2, Fisher’s PLSD for each incubation, no statistically significant differences). No statistically significant differences could be detected between the mucin preparations tested (Fisher’s PLSD). The duration of incubation, however, affected bacterial growth after subculture in TSB: 5 minutes of incubation had no effect (ANOVA, differences between the six isolates tested p<0.001, but no effect of incubation with mucin). Thirty minutes of exposure to mucin containing medium had little if any effect (p=0.008 PLSD (after incubation, 24 hours), while 4 and 8 hour incubations inhibited growth after expansion in fresh TSB (Fig 2, significant differences, p<0.05% 4 and 24 hours after subculture in fresh TSB, Fisher’s PLSD). Some isolates were significantly inhibited even 24 hours after expansion.
Figure 2.
Effect of mucins on bacterial proliferation. The effect of mucin on bacterial replication is expressed as mean differences (SE) between the optical densities of control cultures (in TSB) and bacteria incubated with mucin. (A, B) The effects of the largest mature mucins (Vo, 1.35–1.4 g/min) on all the commensal isolates after 30 minutes and 4 hour incubations, respectively. Examples of individual isolates. (C) Isolate 4; 30 minute incubation, (D) isolate 2; 4 hour incubation) emphasise the similar effect of mucins with different glycosylation densities on bacterial growth. The density of glycosylation increases with increased buoyant density.
Mucinolytic activity
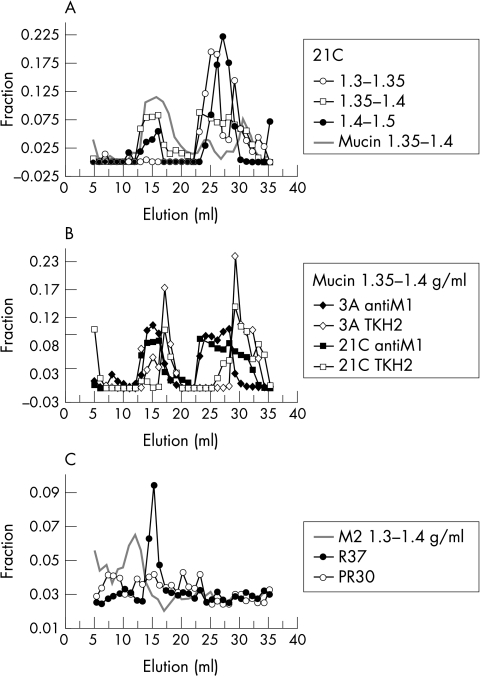
Potential mucinolytic activity was assessed by incubating purified ocular mucins in PBS with bacteria subcultured from a single colony originating from the ocular surface or a contact lens. Size exclusion chromatography was used to assess the ability of ocular surface bacteria to cleave mucin peptide cores or oligosaccharides. Immunoreactivity patterns with antibody antiM1 to MUC5AC peptide core and TKH2 to the oligosaccharide epitope sialylTn were followed after a 16 hour incubation with bacteria at 35°C. In control samples, mucins incubated without protection from enzymatic degradation, a peak of reactivity with antiM1 and TKH2 was observed in the terminal volume, Vt, which represents small hydrodynamic volume molecules (Fig 3), whereas it had not been present in the original Vo mucins. After exposure to bacteria we detected a shift towards intermediate (Vi <4 × 106) and small (Vt = 5× 105 Da) hydrodynamic volumes in both antiM1 (Fig 3A) and TKH2 (Fig 3B) profiles. Some mucins remained, however, in the excluded fraction. AntiM1 positive but TKH2 negative molecules could be detected (Fig 3B) only after incubation with bacteria, and not in control samples. Contact lens isolates displayed more varied patterns, as illustrated in Figure 3C. Incubation with isolate R37, which contained coagulase negative Staphylococcus spp and Pseudomonas spp, resulted in a shift from Vo to a single peak of intermediate size, Vi, while the isolates R30, which contained Bacillus spp and coagulase negative Staphylococcus spp, and 21C (coagulase negative Staphylococcus spp) resulted in a diffuse profile of elution. The patterns in Figure 3 may underestimate proteolytic degradation, since some intracellular ocular mucins remain in the excluded volume after exhaustive reduction and alkylation.31
Figure 3.
Degradation of mucins incubated with bacteria. Changes in the hydrodynamic volume of mucins were assessed by gel filtration after 16 hour incubation with bacteria. Mucins maintained in the same conditions, but without bacteria served as controls. The cross reactions: fractions(a) = (cross reaction(a)/Σ cross reactions) are presented as fractions of the total cross reaction of the eluent with the chosen reagent. Anti-M1 cross reaction after incubation with ocular surface isolate 21C. This cross reaction highlights proteolytic cleavage in MUC5AC mucins that occurs irrespective of mucin buoyant density. Vo mucin 1.35–1.4 g/ml after incubation with ocular surface isolates 3A and 21C: cross reaction with antiM1 and TKH2 to sialylTn. TKH2 negative MUC5AC mucins are obtained in each case. Contact lens adhering mucin buoyant density 1.3–1.4 g/ml incubated with contact lens derived isolates R37 and R30: cross reaction with antiM1. This mucin fraction was obtained after extraction with 10 mM DTT that solubilises aggregates.
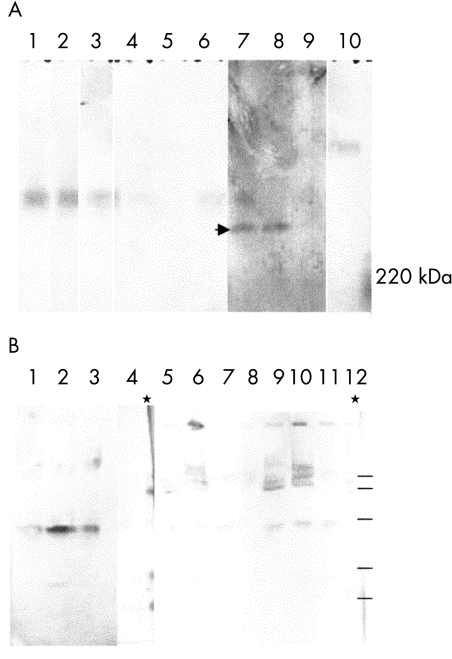
Agarose electrophoresis confirmed the persistence of mucins equimobile with controls (Fig 4A, lanes 1–3) after incubation with isolate 21C (lanes 4–6). Higher mobility species were also observed—for example, after incubation with isolate 3A (Fig 4A, lanes 7–9). Bacterial homogenates had a different mobility from mucins (Fig 4A, lane 10). Electroblots of polyacrylamide gels (Fig 4B) indicated the presence of a glycoprotein band between 66 and 46 kDa in all mucin/bacteria mixtures, which was not seen in control mucin samples (for example, lane 4). WGA positive material is evident in the wells at the top of the gel and in several bands within the stacking gel, confirming the presence of large mucins after bacterial digestion.
Figure 4.
Electrophoretic profiles of mucins incubated with bacteria. (A) Agarose gel electrophoresis of mucins 1.3–1.35 g/ml, followed by vacuum blotting on Immobilon and cross reaction with WGA. Lanes 1–3: Vo, Vi, Vt controls; lanes 4–6 Vo, Vi, Vt incubated with 21Ch; lanes 7–9: Vo, Vi, Vt incubated with 3A; lane 10 bacteria. Arrowhead indicates the novel high mobility degradation bands. (B) Polyacrylamide gel electrophoresis followed by electroblotting on Immobilon and cross reaction with WGA. Lanes 1–8 mucins 1.3–1.35 g/ml: Vi incubated with 1B (lane 1), 3A (lane 2), and 21Ch (lane 3); Vi control and rainbow markers (lane 4); Vt incubated with 1B (lane 5), 3A (lane 6), and 21Ch (lane 7), Vt control (lane 8). Lanes 9–12: mucin 1.2–1.3 g/m Vo: incubated with 1B (lane 9), 3A (lane 10) and 21Ch (lane 11), Vo control (lane 12). The 1.2–1.3 g/ml buoyant density would contain glycoproteins other than mucins. This blot indicates that after hydrodynamic volume fractionation few non-mucin glycoproteins are present in the Vo.
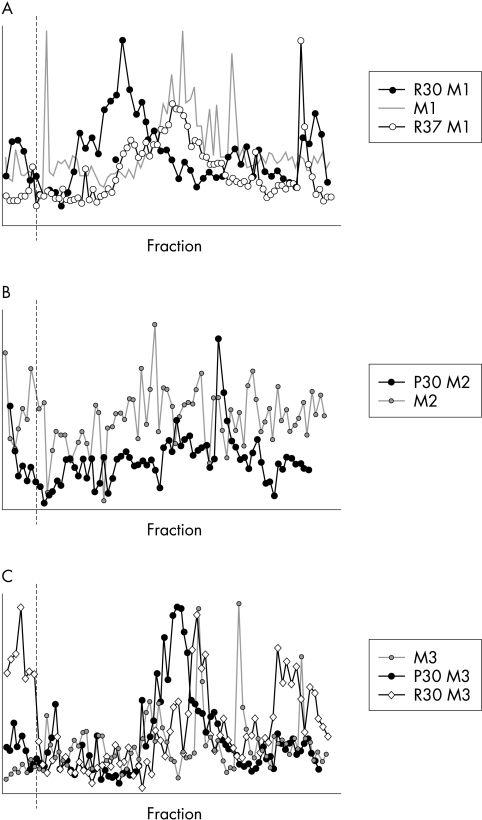
Alterations in mucin glycosylation following incubation with bacteria were also assessed by surveying the charge distributions of mucin subunits. To optimise the detection of bacterial action, mucins with mature glycosylation (1.35–1.4 g/ml buoyant density) were chosen. Reduction and alkylation was performed after incubation with bacteria. In general, subunits with low negative charge were more evident after incubation with bacteria, as shown in Figure 5 for contact lens adherent mucins. Concomitant with the shift to lower charge, soluble contact lens mucins contained a subpopulation of high negative charge not present in the control mucins incubated without bacteria (Fig 5A). Contact lens mucins obtained after one DDT solubilisation exhibited a single charge peak after bacterial digestion, contrasting with the diffuse spread of the parent sample (Fig 5B). Mucins obtained from contact lenses after a second DTT extraction contained a high negative charge population, which disappeared after incubation with bacteria (Fig 5C).
Figure 5.
Ion exchange chromatography on MonoQ of contact lens extracted Vo mucins of buoyant density 1.35–1.4 g/ml after incubation with isolates from contact lenses worn by asymptomatic patients. First extraction mucins are soluble in GuHCl, second and third extractions were performed in the presence of DTT. (A) First extraction control mucins (M1, grey) and M1 after incubation with isolates R30 (solid symbols) and R37 (open symbols). (B) Second extraction control mucins (M2, grey circles) and M2 after incubation with isolate P30. (C) Third extraction control mucins (M3, grey circles) and M3 after incubation with isolates P30 (solid symbols) and R30 (open diamonds).
DISCUSSION
The ocular surface has a number of mechanisms that diminish the bacterial load, among them antimicrobial peptides and blinking. In other mucosae there are complex interactions between bacteria and mucin, not least in that commensal flora participate in mucin turnover. To assess the likelihood of bacteria fulfilling a similar role in the eye, we harvested bacteria from healthy ocular surfaces and assessed their ability to grow on and to modify human ocular mucins.
Though the whole thickness of the preocular mucus down to superficial cells adhere to impression cytology filters, the bacterial yield was similar to that obtained through other harvesting methods.3,32 This suggests that aerobic bacteria either do not penetrate into the mucus gel or lose the ability to grow in culture once trapped in the preocular fluid. The inhibition of bacterial growth after incubation with mucin and the “releasing” effect of NAcCys, are consistent with the latter hypothesis.
Though culture negativity is common, some eyes support a bacterial flora without detriment to their health, as also shown in this study. A stringent sampling method increased the proportion of culture positive lenses, in agreement with earlier results of uneventful contact lens wear.20,32,33 A connection between tear film elements and bacterial adhesion is suggested by the growth of additional colonies after mechanical or enzymatic treatment. Rubbing during lens cleaning is advised by most manufacturers. Addition of NAcCys to cleaning solutions might also decrease bacterial loads on contact lenses.
Mucinolytic activity included both proteolysis and glycolysis. Hydrodynamic volumes were decreased (Fig 3). Fragmentation patterns differed among bacterial isolates (Figs 4 and 5) but appeared insensitive to the degree of glycosylation of the mucins (buoyant density) or to changes in their structure as a result of residence in the tear film and adhesion to lenses. The instability of purified control mucin molecules is suggestive of the physiological role of mucin aggregation with small peptides. The latter would have been dissociated during mucin purification. It might be of interest to mention the paradoxical effect of isolate R37, which was identified as Pseudomonas spp. Its effect on the mucins was slight, perhaps because it also created a microenvironment which inhibited other bacterial enzymes. Loss of sialylTn epitopes (Fig 3C), and the decrease in the net molecular charge (Fig 5), suggest sialidase and sulphatase activity in commensal bacteria. Intracellular MUC5AC glycoforms with highest negative charge are sulphated, while intermediate negative charge is conferred by sialic acids.28 The high negative charge forms could be enriched by the resistance to bacterial enzymes that is conferred by dense glycosylation and sulphation.
Mucin entrapment of bacteria, highlighted by the need for mechanical and enzymatic treatment to release adhering organisms, is complemented by an inhibition of bacterial multiplication that is exerted by both intact and degraded mucins. On the other hand, a reduction in molecular charge is likely to enhance self association of mucins34 resulting in their clearance from the ocular surface.
Acknowledgments
Dr John Leeming, Department of Microbiology, Bristol Royal Infirmary, is thanked for valuable advice on microbiological techniques. Dr Muna Al-Muttawa is thanked for enthusiastic help with these studies. Discussions with Dr AP Corfield have improved the experimental approach and sharpened the analysis of results. Jaques Bara is gratefully acknowledged for the gift of antiM1 antibodies. This project was supported by the Guide Dogs for the Blind Association, UK.
REFERENCES
- 1.Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host-microbial relationship in the intestine. Science 2001;291:881–4. [DOI] [PubMed] [Google Scholar]
- 2.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 1999;285:732–6. [DOI] [PubMed] [Google Scholar]
- 3.Willcox MDP, Stapleton F. Ocular bacteriology. Med Microbiol Rev 1996;7:123–31. [Google Scholar]
- 4.Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. Brit J Ophthalmol 1999;83:737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNamara NA, Van R, Tuchin OS, et al. Ocular surface epithelia express mRNA for human beta defensin-2. Exp Eye Res 1999;69:483–90. [DOI] [PubMed] [Google Scholar]
- 6.Paulsen FP, Pufe T, Schaudig U, et al. Detection of natural peptide antibiotics in human nasolacrimal ducts. Invest Ophthalmol Vis Sci 2001;42:2157–63. [PubMed] [Google Scholar]
- 7.Mantle M, Husar SD. Binding of Yersinia enterocolitica to purified, native small intestinal mucins from rabbits and humans involves interactions with the mucin carbohydrate moiety. Infect Immun 1994;62:1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajjan US, Forstner JF. Role of a 22-kilodalton pilin protein in binding of Pseudomonas cepacia to buccal epithelial cells. Infect Immun 1993;61:3157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuter J, Hatcher VB, Lowy FD. Staphylococcus aureus binding to human nasal mucin. Infect Immun 1996;64:310–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Rayment SA, Gyurko C, et al. The recombinant N-terminal region of human salivary mucin MG2 (MUC7) contains a binding domain for oral Streptococci and exhibits candidacidal activity. Biochem J 2000;345:557–64. [PMC free article] [PubMed] [Google Scholar]
- 11.Marty N, Pasquier C, Dournes JL, et al. Effects of characterised Pseudomonas aeruginosa exopolysaccharides on adherence to human tracheal cells. J Med Microbiol 1998;47:129–34. [DOI] [PubMed] [Google Scholar]
- 12.Prakobphol A, Tangemann K, Rosen SD, et al. Separate oligosaccharide determinants mediate interactions of the low-molecular-weight salivary mucin with neutrophils and bacteria. Biochemistry 1999;38:6817–25. [DOI] [PubMed] [Google Scholar]
- 13.Bosch JA, deGeus EJC, Ligtenberg TJM, et al. Salivary MUC5B-mediated adherence (ex vivo) of Helicobacter pylori during acute stress. Psychosom Med 2000;62:40–9. [DOI] [PubMed] [Google Scholar]
- 14.Bravo JC, Correa P. Sulphomucins favour adhesion of Helicobacter pylori to metaplastic gastric mucosa. J Clin Pathol 1999;52:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davril M, Degroote S, Humbert P, et al. The sialylation of bronchial mucins secreted by patients suffering from cystic fibrosis or from chronic bronchitis is related to the severity of airway infection. Glycobiology 1999;9:311–21. [DOI] [PubMed] [Google Scholar]
- 16.Nordman H, Boren T, Davies JR, et al. pH-dependent binding of Helicobacter pylori to pig gastric mucins. FEMS Immun Med Microbiol 1999;24:175–81. [DOI] [PubMed] [Google Scholar]
- 17.Fleiszig SMJ, Zaidi TS, Ramphal R, et al. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect Immun 1994;62:1799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pflugfelder SC, Liu ZG, Munroy D, et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci 2000;41:1316–26. [PubMed] [Google Scholar]
- 19.Berry M, Harris A, Corfield AP. Mucins adhering to contact lenses. Cambridge: ICRF, 2000.
- 20.Willcox MDP, Power KN, Stapleton F, et al. Potential sources of bacteria that are isolated from contact lenses during wear. Optom Vis Sci 1997;74:1030–8. [DOI] [PubMed] [Google Scholar]
- 21.Hart DE, Hosmer M, Georgescu M, et al. Bacterial assay of contact lens wearers. Optom Vis Sci 1996;73:204–7. [DOI] [PubMed] [Google Scholar]
- 22.Chen FS, Maurice DM. The pH of precorneal tear film and under a contact lens measured with a fluorescent probe. Exp Eye Res 1990;50:251–9. [DOI] [PubMed] [Google Scholar]
- 23.Cowell BA, Willcox MD, Schneider RP. A relatively small change in sodium chloride concentration has a strong effect on adhesion of ocular bacteria to contact lenses. J Applied Microbiol 1998;84:950–8. [DOI] [PubMed] [Google Scholar]
- 24.Berry M, Ellingham RB, Corfield AP. Polydispersity of normal human conjunctival mucins. Invest Ophthalmol Vis Sci 1996;37:2559–71. [PubMed] [Google Scholar]
- 25.Carlstedt I, Hermann A, Karlsson H, et al. Characterization of two different glycosylated domains from the insoluble mucin complex of rat small intestine. J Biol Chem 1993;268:18771–81. [PubMed] [Google Scholar]
- 26.Corfield AP, Carrington SD, Hicks SJ, et al. Ocular mucins: purification, metabolism and function. Progress Ret Eye Res 1997;16:627–56. [Google Scholar]
- 27.Berry M, Ellingham RB, Corfield AP. Membrane-associated mucins in normal human conjunctiva. Invest Ophthalmol Vis Sci 2000;41:398–403. [PubMed] [Google Scholar]
- 28.Ellingham RB, Berry M, Stevenson D, et al. Secreted human conjunctival mucus contains MUC5AC glycoforms. Glycobiology 1999;9:1181–9. [DOI] [PubMed] [Google Scholar]
- 29.Bara J, Chastre E, Mahiou J, et al. Gastric M1 mucin, an early oncofetal marker of colon carcinogenesis, is encoded by the MUC5AC gene. Int J Cancer 1998;75:767–73. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi H, Tergo T, Kowashima Y. Serum sialylTn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J Clin Oncol 1992;10:95–101. [DOI] [PubMed] [Google Scholar]
- 31.Berry M, Ellingham RB, Corfield AP. Conjunctiva: organ and cell culture. In: Jones GE, eds. Human cell culture protocols. Totowa, New Jersey: Humana Press; 1996:503–17. [DOI] [PubMed]
- 32.Larkin DF, Leeming JP. Quantitative alterations of the commensal eye bacteria in contact lens wear. Eye 1991;5:70–4. [DOI] [PubMed] [Google Scholar]
- 33.Larkin DF, Kilvington S, Easty DL. Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br J Ophthalmol 1990;74:133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bromberg LE, Barr DP. Self-association of mucin. Biomacromolecules 2000;1:325–34. [DOI] [PubMed] [Google Scholar]