Abstract
Aims: To localise Smads3/4 proteins in lens epithelial cells (LECs) of fresh and postoperative human specimens. Smads3/4 are involved in signal transduction between transforming growth factor β (TGFβ) cell surface receptors and gene promoters. Nuclear localisation of Smads indicates achievement of endogenous TGFβ signalling in cells.
Methods: Three circular sections of the anterior capsule, one lens, and 17 capsules undergoing postoperative healing were studied. Immunohistochemistry was performed for Smads3/4 in paraffin sections of the specimens. The effect of exogenous TGFβ2 on Smad3 subcellular localisation was examined in explant cultures of extracted human anterior lens epithelium.
Results: The cytoplasm, but not the nuclei, of LECs of uninjured lenses was immunoreactive for Smads3/4. In contrast, nuclear immunoreactivity for Smads3/4 was detected in LECs during capsular healing. Nuclei positive for Smads3/4 were observed in monolayered LECs adjacent to the regenerated lens fibres of Sommerring’s ring. Interestingly, the nuclei of LECs that were somewhat elongated, and appeared to be differentiating into fibre-like cells, were negative for Smads3/4. Fibroblast-like, spindle-shaped lens cells with nuclear immunoreactivity for nuclear Smads3/4 were occasionally observed in the extracellular matrix accumulated in capsular opacification. Exogenous TGFβ induced nuclear translocation of Smad3 in LECs of anterior capsule specimens in explant culture.
Conclusions: This is consistent with TGFβ induced Smad signalling being involved in regulating the behaviour of LECs during wound healing after cataract surgery.
Keywords: lens epithelial cell, transforming growth factor β, Smad, cataract surgery
Cytokines are thought to orchestrate the behaviour of lens epithelial cells during healing after cataract extraction. In many cases this results in aberrant growth of residual lens epithelial cells and the formation of fibrotic scar tissue (posterior capsular opacification, also referred to as secondary cataract or after-cataract).1–6 Transforming growth factor β (TGFβ), mainly TGFβ2, is abundant in the aqueous humour.7–9 TGFβ is pivotal in regulating proliferation, differentiation, and extracellular matrix (ECM) expression by cells in a positive or negative manner.10,11 Lens cells express TGFβ isoforms and TGFβ receptors.12–15 Moreover, we have reported that human lens epithelial cells and macrophages adhering to implanted intraocular lenses (IOLs) express TGFβ family members.15–17
Smads are proteins involved in mediating intracellular signal transduction between TGFβ/bone morphogenic protein (BMP) receptors and gene promoters,18,19 although other signalling pathways have also been reported.20 On ligand binding to the TGFβ receptor, phosphorylated Smad2 or Smad3 translocates to the nucleus in a complex with Smad4, and binds to a site in a promoter region. We observed that Smads3/4 translocate to the nuclei of lens epithelial cells in the healing murine lens following an anterior capsular injury. This is also the case in lens epithelial cells from murine lenses organ cultured in the presence of TGFβ2. Taken together this indicates that healing murine lens cells are regulated by endogenous TGFβ2 through the Smad system.14 We hypothesised that human lens epithelial cells are also regulated by endogenous TGFβ during healing following cataract extraction and implantation of an IOL. To explore this hypothesis, in the present study we examined Smads3/4 localisation in lens cells in postoperative lens capsule specimens from humans. We also examined phenotypic alterations in lens cells by immunodetection of β-crystallin and α-smooth muscle actin (αSMA), these being key markers for lens fibres and myofibroblastic cells respectively.21–23
MATERIALS AND METHODS
Specimens
All specimens examined had been removed from Japanese patients, with a mean age of 64 years (range 28–80; Table 1). Specimens were obtained at the Wakayama Medical College Hospital, Wakayama, Japan, or were supplied by the IOL Implant Data System Committee of the Japanese Society of Cataract and Refractive Surgery. The specimens were fixed immediately after the removal. They were processed for histological examination as follows after informed consent was obtained. Circular sections of the anterior capsule of cases 1–3 were obtained during cataract surgery. The crystalline lens of case 4 was extracted because of its dislocation and was removed from an enucleated globe. Anterior capsular specimens with anterior subcapsular cataract were excluded in this study. Cases 5–21 were capsules in various stages of postoperative healing obtained during a subsequent operation to treat proliferative vitreoretinopathy, dislocation of the capsular bag with an IOL, or malignant glaucoma. Cases 6 and 8 were postoperative capsules without IOL implantation. Cases 7 and 16 had undergone implantation of an Acrysof IOL (Alcon, Fort Worth, TX, USA) and a silicone IOL, respectively. Other postoperative specimens had a polymethylmethacrylate IOL. Specimens were fixed in 10% formalin and embedded in paraffin as previously reported.24
Table 1.
Summary of the cases and the results
| Presence/absence of nuclear Smads3/4 positive cells | ||||||||
| Epithelial-type cells adjacent to regenerated lens fibres in Sommerring’s ring | Cells amid fibrous matrix accumulation | |||||||
| Case No | Age* | Sex | Duration | Cause† | Smad3 | Smad4 | Smad3 | Smad4 |
| 1 | 65 | F | − | CCC‡ | − | − | − | − |
| 2 | 72 | M | − | CCC | − | − | − | − |
| 3 | 58 | M | − | CCC | − | − | − | − |
| 4 | 72 | F | − | Lens dislocation | − | − | − | − |
| 5 | 28 | M | 6 days | IOL dislocation | + | + | NE | NE |
| 6 | 64 | M | 10 days | PVR | + | + | NE | NE |
| 7 | 58 | F | 14 days | PVR | + | + | NE | NE |
| 8 | 68 | F | 0.65 y | PVR | + | + | + | + |
| 9 | 75 | F | 0.75 y | PVR | + | + | + | + |
| 10 | 51 | M | 1 y | Malignant glaucoma | + | + | + | + |
| 11 | 53 | M | 3 y | PVR | + | + | NE | NE |
| 12 | 75 | M | 2.7 y | IOL dislocation | + | + | + | + |
| 13 | 78 | M | 4 y | PVR | + | + | + | + |
| 14 | 80 | M | 4 y | PVR | + | + | + | + |
| 15 | 76 | M | 4.6 y | IOL dislocation | + | + | + | + |
| 16 | 68 | M | 5 y | IOL dislocation | + | + | + | + |
| 17 | 77 | F | 5.4 y | PVR | + | + | + | + |
| 18 | 77 | F | 6.2 y | IOL dislocation | − | − | + | + |
| 19 | 60 | M | 8 y | IOL dislocation | − | − | NE | NE |
| 20 | 68 | M | 9 y | IOL dislocation | + | + | NE | NE |
| 21 | 31 | M | 10 y | IOL dislocation | − | − | − | − |
*Age at the removal of the intraocular lens (IOL); † duration between implantation and explantation of the IOL; y = year(s); ‡CCC = continuous circular capsulorhexis; PVR = IOL removal to obtain the better observation of the fundus during vitrectomy for proliferative vitreoretinopathy; capsulotomy = obtained by anterior capsulotomy during cataract surgery; − = negative; + = positive (regardless the incidence of positive cells); NE = not examined.
Immunohistochemistry
Deparaffinised sections cut at 5 μm thickness were immunostained with rabbit polyclonal anti-Smad3 antibody (diluted × 100 in phosphate buffered saline (PBS); Zymed, South San Francisco, CA, USA), goat polyclonal antibody against Smad4 (diluted × 200 in PBS; Santa Cruz Biotechnology, Santa Cruz, CA, USA), monoclonal anti-αSMA antibody (×100, Sigma, Saint Louis, MI, USA) or rabbit polyclonal anti-β-crystallin antibody (McAvoy, 1978, 5 μg/ml in PBS). After washing in PBS, specimens were allowed to react with peroxidase conjugated polyclonal secondary antibodies (×200 in PBS; Cappel, Organon-Teknika, West Chester, PA, USA). Reactivity/specificity of the antibodies used here was referred to the previous publication by Flanders et al.25 After another wash, the reaction product was visualised with 3,3’-diaminobenzidine and sections were counterstained with methyl green and mounted in balsam. Negative control staining was performed with rabbit, goat or mouse non-immune IgG as primary antibody, as appropriate.
Explant culture experiment
In order to define whether nuclear translocation of Smad3 is induced by TGFβ2, we cultured the anterior capsular sections excised during cataract surgery in the presence and absence of TGFβ2. An anterior capsule was put into the Eagle’s medium (Gibco BRL, Life Technologies Inc, Gaithersberg, MD, USA) in the presence or absence of the active form of porcine TGFβ2 (1.0 ng/ml, R & D system, Minneapolis, MN, USA) immediately after extraction. We have confirmed that this TGFβ2 is effective to human cells in vitro by determining collagen I upregulation in human subconjunctival fibroblast culture. Altogether 15 specimens were studied, the ages ranged from 58 to 80 years of age and the mean age was 67.7 (SD 6.31) years; the ratio of male to female specimens was 8 to 7. Capsular specimens with anterior subcapsular cataract were excluded from this study. After incubation at 37°C in 5.0% carbon dioxide/95.0% air for 0 (n=2), 30 minutes (n=3), 60 minutes (n=3), 3 (n=2), 4 (n=3), and 24 hours (n=2), the specimens were fixed in 2.0% paraformaldehyde in 0.1M phosphate buffer for 24 hours. Then the specimens was routinely embedded in paraffin. Deparaffinised sections 5.0 μm thick were immunostained for Smad3 with a polyclonal antibody (Zymed) as described above. After counterstaining in methyl green, the sections were mounted in balsam after dehydration.
RESULTS
As shown in Table 1 we obtained specimens for analysis from patients at the time of cataract surgery (cases 1–4) and at various times after cataract surgery (cases 5–21). This allowed us to analyse the distribution of TGFβ signalling molecules, the Smads, in relation to postoperative cellular changes. In particular, we examined Smads 3/4 localisation patterns in cells of the epithelium, Sommerring’s ring and the ECM accumulated next to the implanted lens optic. In general, we noted that, beginning at 10 days after surgery, indications of lenticular fibre regeneration (Sommerring’s ring) could be observed in the peripheral capsular bag. Fibroblast-like spindle-shaped lens cells were commonly observed in areas of fibrous opacification of the capsule in the specimens obtained at or beyond 0.65 year after initial surgery. Histology was similar to that previously reported.13
Epithelial cells and cells adjacent to Sommerring’s ring
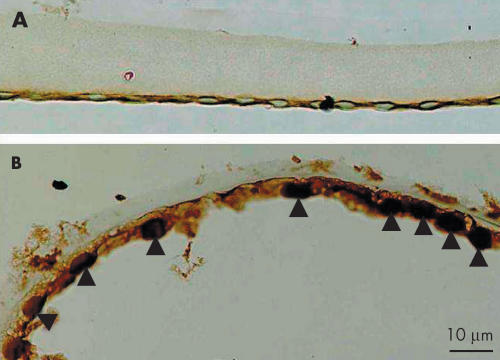
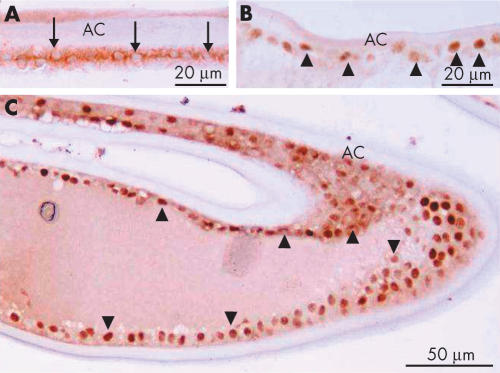
To determine if there was any evidence of TGFβ signalling through the Smad pathway as a result of cataract surgery, we compared the intracellular localisation of Smads in epithelial cells excised at the time of cataract surgery with epithelial cells in specimens collected postoperatively. Smad3, a TGFβ signalling Smad, was detected in the nuclei of epithelial-shaped lens cells as early as 10 days postoperatively, whereas it was negative in the nuclei of freshly isolated lens epithelia (Fig 1).
Figure 1.
Intracellular immunolocalisation of Smad3 in human lens epithelial cells on the capsular specimen obtained during cataract surgery (A, case 2) or extracted 10 days after cataract surgery (B; case 6). Lens epithelial cells on the capsular specimen, obtained during first cataract surgery are immunoreactive for Smad3 in the cytoplasm, but not in the nuclei. On the other hand, lens cells 10 days postoperatively show nuclear immunoreactivity for Smad3 with a weak reaction in the cytoplasm. Indirect immunostaining. Bar, 10 μm.
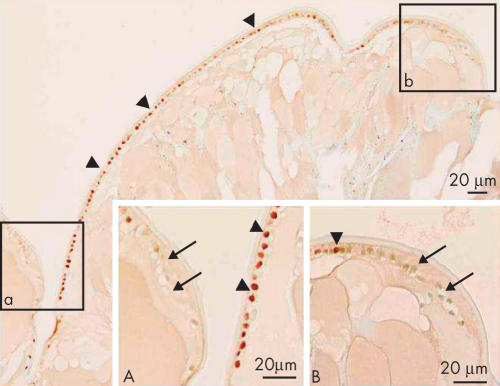
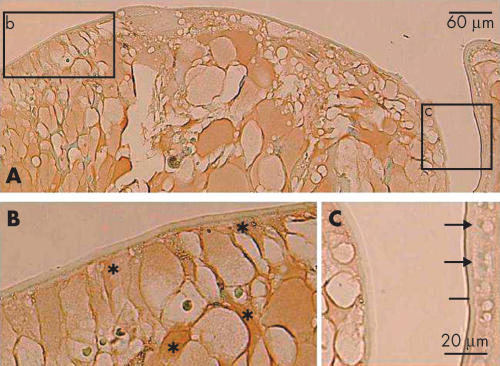
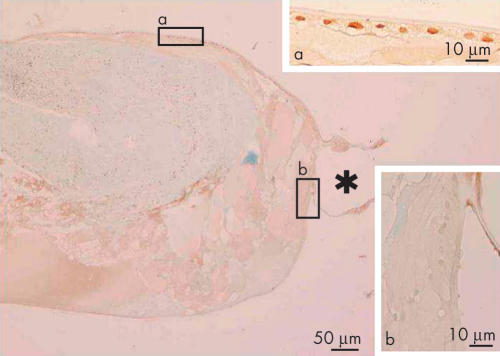
At 2.7 years postoperatively, in the area of the peripheral capsular bag, cuboidal, epithelial-like lens cells were positive for nuclear Smad3. Adjacent cells that were somewhat elongated, lacked immunoreactivity for Smad3 in their nuclei (Fig 2). The cuboidal cells between the regenerated fibre-like cells and residual capsule appeared morphologically similar to lens epithelial cells by light microscopy, whereas the elongated cells looked like epithelial cells undergoing the transition into fibres. These epithelial and fibre-like phenotypes were consistent with the distribution of fibre specific β-crystallin; cuboidal cells were negative for β-crystallin, whereas both denucleated lenticular fibres and elongated cells containing nuclei were immunoreactive for β-crystallin (Fig 3). Five years postoperatively, the cuboidal, epithelial-like lens cells maintained their nuclear Smad3 immunoreactivity and a similar loss of nuclearly localised Smad3, as described above, was evident in the differentiating fibre-like cells (Fig 4).
Figure 2.
Immunolocalisation of Smad3 in lens cells in Sommerring’s ring 2.7 years postoperatively (case 12). Lens epithelial cells beneath the capsule (arrowheads) are positive for nuclear Smad3. (A) and (B) Higher magnification pictures of boxed areas a and b, respectively, show the progressive disappearance of nuclear Smad3 in elongating lens cells (arrows) as they differentiate into the fibre-like cells. Indirect immunostaining. Bar, 20 μm.
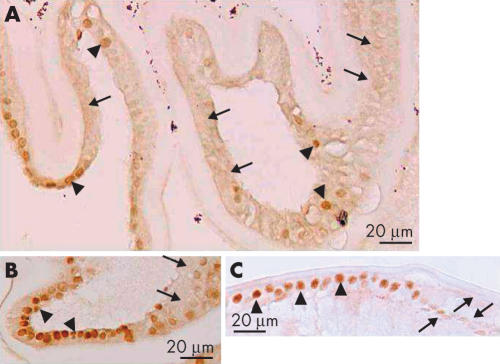
Figure 3.
Immunolocalisation of β-crystallin in Sommerring’s ring 2.7 years postoperatively (case 12). (A) Enlarged/elongated lens cells forming the Sommerring’s ring structure are immunostained for β-crystallin, whereas monolayered epithelial-shaped lens cells are unstained; (B) and (C) are the high magnification pictures of boxed areas b and c, respectively. Asterisks in (B) show immunostained enlarged/elongated cells reactive for fibre specific β-crystallin. Arrows in (C) show a monolayer of unstained lens cells. Indirect immunostaining. Bar, 60 μm (A); 20 μm (B and C).
Figure 4.
Immunolocalisation of Smad3 in lens cells in Sommerring’s ring 5 years postoperatively (case 16). Lens epithelial cells beneath the anterior capsule (boxed area a) are positive for nuclear Smad3, but somewhat elongated lens cells in the equator (boxed area b) do not show nuclear immunoreactivity. The asterisk indicates the position of the haptic loop of the intraocular lens. Indirect immunostaining. Bar, 50 μm; inserts bar, 10 μm.
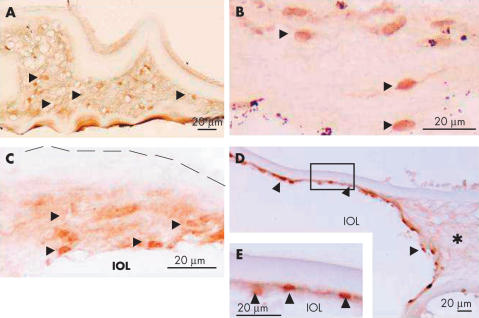
Smad4 is the member of Smad family of proteins which works in both TGFβ and BMP signal transduction pathways. Similar to smad3, Smad4 was detected in the cytoplasm, but not in the nuclei, of lens epithelial cells lining the inner surface of the uninjured anterior lens capsule (Table 1, Fig 5A). Postoperatively, Smad4 was located in the nuclei of cuboidal lens epithelial cells between the anterior and posterior capsules in the peripheral capsular bag and in lens epithelial cells adjacent to the regenerated lenticular structure (Fig 5B, C). Smad4 was also detected in most cell nuclei of cuboidal lens cells in the capsular bag, but not in the nuclei of the somewhat elongated lens cells that appear to be undergoing differentiation into lenticular fibre-like cells (Fig 6).
Figure 5.
Intracellular immunolocalisation of Smad4 in lens epithelial cells following cataract surgery. (A) (Case 1), epithelial cells (arrows) in an uninjured lens show Smad4 immunoreactivity in the cytoplasm. In (B) a specimen obtained 6 days after cataract surgery (case 5), some of lens epithelial cells show Smad4 in the nuclei (arrowheads), while others do not. (C) Nuclei of the lens epithelial cells (arrowheads) in the closed capsular bag 1 year postoperatively exhibit nuclear Smad4 immunoreactivity (case 10). AC = anterior capsule. Indirect immunostaining. Bar, 20 μm (A) and (B), and 50 μm (C).
Figure 6.
Reduction of nuclear immunoreactivity of Smad4 in elongating lens cells. In (A) (case 9), some of the lens cells are positive for nuclear Smad4 (arrowheads), whereas others are negative for nuclear Smad4 (arrows). In (B) (case 7) and (C) (case 20), the cells with strong nuclear immunoreactivity tend to be cuboidal (arrowheads) whereas in regions where the cells appear to be elongating, Smad4 immunoreactivity tends to be absent or reduced (arrows). Indirect immunostaining. Bar, 20 μm.
Lens epithelial cells in specimens obtained 6.2, 8, and 10 years postoperatively or later did not show positive immunoreactivity for nuclear Smads (Table 1).
Fibroblast-like lens cells amid extracellular matrix (ECM) accumulation
Fibroblastic, spindle-shaped lens cells with nuclear immunoreactivity for Smads3/4 were observed in regions of ECM accumulation (Fig 7A–C). Although cells with unstained nuclei were also present amid matrix, the majority of the fibroblast-like lens cells there were positive for αSMA as previously reported (Fig 8).13 In specimens with an IOL, which was associated with ECM accumulation, however, cells with nuclear Smad3 (not illustrated) and Smad4 (Fig 7D, E) were prominent in the fibrous tissue adjacent to the optic portion of the IOL, while most elongated lens cells within the ECM accumulation did not stain for nuclear Smads3/4. Fibroblastic lens cells in specimens obtained 8 and 10 years postoperatively or later did not show positive immunoreactivity for nuclear Smads (Table 1).
Figure 7.
Intracellular immunolocalisation of Smads3/4 in lens cells amid ECM on the healing capsules. (A) Lens cells embedded in ECM exhibit nuclear immunoreactivity for Smad3 (arrowheads) in specimens from case 9 (0.75 years postoperatively). (B) Lens cells with nuclei positive for Smad4 (arrowheads) amid extracellular matrix accumulated inside the capsule of the specimen (case 9). (C) Some lens cells within the ECM show Smad4 immunoreactivity in nuclei (arrowheads), while others exhibit only faint cytoplasmic immunoreactivity. Lens cells attached to the intraocular lens (IOL) optic tend to exhibit the strongest nuclear Smad4 staining (case 13). (D) Nuclei of lens cells in the ECM that has accumulated around the optic portion of an IOL still stain for Smad4 2.7 years after surgery (arrowheads, case 12). No nuclear Smad4 positive cells are seen in the ECM (asterisk) that is not attached to the lens optic. (E) Shows increased magnification of the lens cells in the boxed area in (D). Broken line in (C) indicates the anterior surface of the anterior capsule. Indirect immunostaining. Bar, 50 μm for (A) and (B) and 20 μm for (C) to (E).
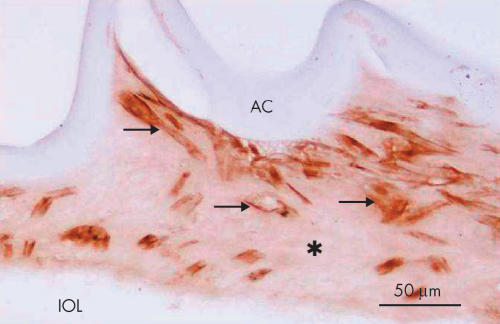
Figure 8.
Expression of α-smooth muscle actin in elongated, (myo)fibroblast-like lens cells (case 15). Almost all the cells with such a configuration (arrows) are positive for α-smooth muscle actin in a filamentous pattern in the cytoplasm. Indirect immunostaining. Asterisk, extracellular matrix accumulation; Bar, 50 μm.
Smad3 translocation in lens epithelial cells cultured with exogenous TGFβ2
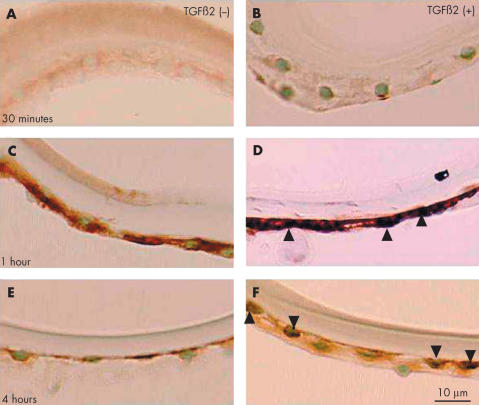
To determine if TGFβ induces nuclear translocation of Smads in vitro, we cultured anterior capsule specimens in the presence or absence of this growth factor. In the absence of TGFβ, Smad3 protein was not detected in the nuclei of lens epithelial cells after 30 minutes, 1 hour, or 4 hours culture (Fig 9A, 9C, 9E). Cytoplasmic immunoreactivity was weak, or absent, after 30 minutes but was particularly strong after 1 hour culture (Fig 9C). In specimens cultured with TGFβ nuclear localisation of Smad3 was evident. Little reactivity was detected after 30 minutes (Fig 9B); however, after 1 hour with TGFβ there was strong nuclear reactivity (Fig 9D). After 4 hours with TGFβ, Smad3 was absent from many nuclei and only weakly detected in others (Fig 9F). Nuclear immunoreactivity was also absent after 3 hours in TGFβ treated specimens (not shown).
Figure 9.
Nuclear translocation of Smad3 in the epithelium of human lens anterior capsule specimens cultured in the presence of exogenous TGFβ2. In controls, cultured in the absence of TGFβs, Smad3 immunoreactivity was weak, or absent, from the cytoplasm of lens epithelial cells after 30 minutes’ incubation (A). Cytoplasmic immunoreactivity was very strong after 1 hour (C) and weak after 4 hour (E) incubation periods. The cells in the presence of TGFβ2 at 30 minutes showed faint nuclear Smad3 (B), whereas those at 1 hour exhibited marked Smad3 nuclear immunoreactivity (arrowheads, D). After 4 hours, weak nuclear immunoreactivity was detected in some cells (arrowheads), but not in others (F). Indirect immunostaining. Bar, 10 μm.
Controls
No specific immunoreactvity was observed in each negative control when non-immune serum was substituted for the primary antibody (not illustrated).
DISCUSSION
Results from the present study indicate that human lens epithelial cells are regulated by endogenous TGFβ during postoperative healing. Nuclear localisation of Smads3/4 was demonstrated in postoperative lenses, while these Smads were not detected in the nuclei of lens epithelial cells of uninjured lenses or in the cells of anterior capsule freshly obtained during ocular surgery. It has long been hypothesised that TGFβs might influence postoperative lens cell behaviour. This is because it was shown that aqueous humour collected at the time of surgery contains abundant TGFβ2,7–9 although the ratio of active/total TGFβ in aqueous humour is reportedly altered during the relatively earlier phase of healing interval following cataract surgery.26 Moreover, we have shown that TGFβ2, but not β1 and β3, is involved in ocular morphogenesis in mice.27 A similar phenomenon was observed by us in injured mouse lenses; in vivo neutralisation of TGFβ2, but not TGFβ1 and TGFβ3, by exogenous antibodies inhibits nuclear translocation of Smad4 during lens wound healing in mice, indicating that endogenous TGFβ2 utilises the Smad signalling pathway during healing of the mouse lens epithelium.14
In Sommerring’s ring of the peripheral capsular bag, lens epithelial cells located between regenerated lenticular fibre-like cells and lens capsule were found to be positive for nuclear Smads3/4. This finding indicates that TGFβ signals are modulating these postoperative lens cells. Although these cells were attached to fibre-like cells and were epithelial-like in morphology similar to the epithelial cells in uninjured lenses, the presence of nuclear Smads3/4 positive lens cells indicates that they may be physiologically and transcriptionally different. Interestingly, the nuclei of elongated, β-crystallin positive, lens fibre-like cells, lacked nuclear immunoreactivity for Smads3/4. This finding might indicate that the TGFβ-Smad signalling is not essential to the differentiation of these fibre-like cells during wound healing. Although there is evidence that TGFβ signalling is required for events in normal fibre differentiation in mice28, as yet there are no indications of whether this process is mediated by Smads. TGFβ is known to activate other signalling pathways; for example, the JNK pathway, rather than the Smad proteins, is involved in the response of muscle cells to TGFβ.29
TGFβ upregulates expression of ECM components in various cell types including lens cells.30,31 We detected accumulation of collagen types, laminin, and fibronectin in human opacified lens capsules with an IOL in the previous study13 and also in injured mouse lenses in a late phase of repair (Saika et al, data submitted). These ECM components are likely to be accumulated by the fibroblast-like lens cells induced by TGFβ. In the present series of human specimens, some fibroblast-like lens cells in the accumulated ECM were positive for nuclear Smad3/4, although many were negative. There was some variation among specimens, in particular, nuclear Smads3/4 positive cells were more common in one specimen extracted at a relatively early phase of healing (0.75 year postoperatively; case 9, Fig 7A). Interestingly, lens cells attached to the IOL were more likely to stain for nuclear Smad4, while many of those within the ECM were unstained (Fig 7C, D). This suggests that attachment to a foreign body, such as an IOL, could influence susceptibility to TGFβ and fibrotic changes. On the other hand, αSMA positive myofibroblastic cells were found to be distributed amid the matrix, indicating that the cells with nuclear Smad and cytoplasmic αSMA did not overlap. One of the explanations may include that nuclear Smads can be degraded by ubiquitine system.32 In this context, further studies on how different materials influence Smad mediated TGFβ signalling in lens cells may prove useful in the construction of more biocompatible IOLs.
The present study also shows that exogenous TGFβ2 induces Smad3 translocation to cell nuclei of organ cultured human lens epithelia, further indicating that TGFβ is capable of translocating Smad3 to human lens cell nuclei. TGFβ2 was added at the concentration of 1.0 ng/ml to the organ culture medium and this corresponds with estimates of physiological levels.9 This is consistent with our conclusion that the presence of Smads in the nuclei of lens cells during postoperative healing indicates that TGFβ is a mediator of this process. However, in the in vitro study, the Smads nuclear translocation was transient and Smads were no longer detected after 3 hours’ incubation. The ubiquitin system reportedly degrades the Smads after transcription has been switched on,32 so this might account for the rapid loss of nuclear reactivity in these cultures. In contrast, the analysis of the in vivo material showed that the cuboidal lens epithelial cells, which lined the regenerated fibre mass, still have Smads3/4 in their nuclei even up to 5.4 years postoperatively. Detection of Smads 3/4 in the nuclei of lens cells for such a long period after surgery is an important finding. It indicates that the lens cells continue to be influenced by TGFβ over an extended period. On the other hand, it is also possible that ongoing ocular diseases—that is, diabetic retinopathy or proliferative vitreoretinopathy, might affect the TGFβ-Smad signalling in lens cells. Moreover, cytoplasmic Smad3 immunoreactivity seemed markedly increased at 1 hour’s culture compared with that in 30 minutes’ culture both in TGFβ2(+) and (−) cultures. Although immunohistochemistry is not a quantitative method, we consider it possible that exogenous TGFβ2 upregulates de novo biosynthesis of Smad3 protein by some stimulation in association with removal of the epithelium from lens cortical fibres. In the absence of exogenous TGFβ2, however, upregulated Smad3 protein might not be phosphorylated and translocated. This should be borne in mind when strategies to protect lens cells from the damaging effects of wound healing, are being considered.
Acknowledgments
The authors thank the members of IOL Implant Data System Committee of the Japanese Society of Cataract and Refractive Surgery for providing specimens.
REFERENCES
- 1.McDonnell PJ, Zarbin MA, Green WR. Posterior capsule opacification in pseudophakic eyes. Ophthalmology 1983;90:1548–53. [DOI] [PubMed] [Google Scholar]
- 2.Kappelhof JP, Vrensen GFJM, de Jong PTVM, et al. An ultrastructural study of Elschnig’s pearls in the pseudophakic eye. Am J Ophthalmol 1986;101:58–69. [DOI] [PubMed] [Google Scholar]
- 3.Apple DJ, Solomon KD, Tetz MR, et al. Posterior capsule opacification. Surv Ophthalmol 1992;37:73–116. [DOI] [PubMed] [Google Scholar]
- 4.Saika S, Ohmi S, Tanaka S, et al. Outgrowth of lens epithelial cells and matrix formation on intraocular lenses in rabbit eyes. J Cataract Refract Surg 1996;22:835–40. [DOI] [PubMed] [Google Scholar]
- 5.Maecock WR, Spalton DJ, Stanford MR. Role of cytokines in the pathogenesis of posterior capsule opacification. Br J Ophthalmol 2000;84:332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wormstone IM, Del Rio-Tsonis K, McMahon G, et al. FGF: an autocrine regulator of human lens cell growth independent of added stimuli. Invest Ophthalmol Vis Sci 2001;42:1305–11. [PubMed] [Google Scholar]
- 7.Jampel HD, Roche N, Stark WJ, et al. Transforming growth factor-beta in human aqueous humor. Curr Eye Res 1990;9:963–9. [DOI] [PubMed] [Google Scholar]
- 8.Cousins SW, McCabe MM, Danielpour D, et al. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci 1991;32:2201–11. [PubMed] [Google Scholar]
- 9.Inatani M, Yanihara H, Katsuta H, et al. Transforming growth factor-β 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol 2001;239:109–13. [DOI] [PubMed] [Google Scholar]
- 10.Massague J. The transforming growth factor-β family. Ann Rev Cell Biol 1990;6:597–641. [DOI] [PubMed] [Google Scholar]
- 11.Miller DA, Pelton RW, Derynck R, et al. Transforming growth factor β: a family of growth regulatory peptides. Ann NY Acad Sci 1990;593:208–17. [DOI] [PubMed] [Google Scholar]
- 12.Gordon-Thomson C, de Iongh RU, Hales AM, et al. Differential cataractogenic potency of TGF-β1, -β2, and -β3 and their expression in the postnatal rat eye. Invest Ophthalmol Vis Sci 1998;39:1399–409. [PubMed] [Google Scholar]
- 13.Saika S, Kawashima Y, Miyamoto T, et al. Immunolocalization of prolyl 4-hydroxylase subunits, α-smooth muscle actin, and extracellular matrix components in human lens capsules with lens implants. Exp Eye Res 1998;66:283–94. [DOI] [PubMed] [Google Scholar]
- 14.Saika S, Okada Y, Miyamoto T, et al. Smad translocation and growth suppression in lens epithelial cells by endogenous TGF-β 2 during wound repair. Exp Eye Res 2001;72:679–86 [DOI] [PubMed] [Google Scholar]
- 15.Saika S, Miyamoto T, Kawashima Y, et al. Immunolocalization of TGF-β1, -β2 and -β3, and TGF-β receptors in human lens capsules with lens implants. Graefes Arch Clin Exp Ophthalmol 2000;238:283–94. [DOI] [PubMed] [Google Scholar]
- 16.Saika S, Miyamoto T, Okada Y, et al. Transforming growth factor-β isoform proteins in cell and matrix deposits on intraocular lenses. J Cataract Refrcat Surg 2000;26:709–15. [DOI] [PubMed] [Google Scholar]
- 17.Saika S, Miyamoto T, Tanaka T, et al. Latent TGFβ binding protein-1 and fibrillin-1 in human capsular opacification and in cultured lens epithelial cells. Br J Ophthalmol 2001;85:1362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massague J. How cells read TGF-β signals. Nature Rev (Mol Cell Biol) 2000;1:169–78. [DOI] [PubMed] [Google Scholar]
- 19.Piek E, Heldin C-H, ten Dijke P. Specificity, diversity, and regulation in TGF-β superfamily signaling. FASEB J 1999;13:2105–24. [PubMed] [Google Scholar]
- 20.Shibuya H, Yamaguchi K, Shirakabe K, et al. TAB1: an activator of the TAK1 MAPKKK in TGF-β signal transduction. Science 1996;272:1179–82. [DOI] [PubMed] [Google Scholar]
- 21.McAvoy JW. Cell division, cell elongation and the co-ordination of crystalline gene expression during lens morphogenesis in the rat. J Embryo Exp Morphol 1978;45:271–8. [PubMed] [Google Scholar]
- 22.Schmitt-Graff A, Pau H, Spahr R, et al. Appearance of alpha-smooth muscle actin in human eye lens cells of anterior capsular cataract and in cultured bovine lens-forming cells. Differentiation 1990;43:115–22. [DOI] [PubMed] [Google Scholar]
- 23.Masur SK, Dewal HS, Dinh TT, et al. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci 1996;93:4219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saika S, Shiraishi A, Saika S, et al. Role of lumican in the corneal epithelium during wound healing. J Biol Chem 2000;275:2607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flanders KC, Kim ES, Roberts AB. Immunohistochemical expression of Smads 1–6 in the 15-day gestation mouse embryo: signaling by BMPs and TGF-βs. Dev Dyn 2001;220:141–54. [DOI] [PubMed] [Google Scholar]
- 26.Wallentin N, Wickstrom K, Lundberg C. Effect of cataract surgery on aqueous TGF-beta and lens epithelial cell proliferation. Invest Ophthalmol Vis Sci 1998;39:1410–8. [PubMed] [Google Scholar]
- 27.Saika S, Saika S, Liu C-Y, et al. TGFβ2 in corneal morphogenesis during mouse embryonic development. Dev Biol 2001;240:419–32. [DOI] [PubMed] [Google Scholar]
- 28.De Iongh RU, Lovicu FJ, Overbeek PA, et al. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development 2001;128: 3995–4010. [DOI] [PubMed] [Google Scholar]
- 29.Rousse S, Lallemand F, Montarras D, et al. Transforming growth factor-beta inhibition of insulin-like growth factor-binding protein-5 synthesis in skeletal muscle cells involves a c-Jun N-terminal kinase-dependent pathway. J Biol Chem 2001;276:46961–7. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Hales AM, Chamberlain CG, et al. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Invest Ophthalmol Vis Sci 1994;35:388–401. [PubMed] [Google Scholar]
- 31.Nishi O, Nishi K, Fujita T, et al. Effects of the cytokines on the proliferation of and collagen synthesis by human cataract lens epithelial cells. Br J Ophthalmol 1996;80:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo RS, Massague J. Ubiquitin-dependent degradation of TGF-β-activated Smad2. Nat Cell Biol 1999;1:472–8. [DOI] [PubMed] [Google Scholar]