Abstract
Aim: To evaluate the safety and efficacy of a new topical cysteamine formulation, stable at room temperature, for the treatment of corneal cystine crystals in cystinosis.
Methods: 20 study subjects were enrolled in the safety study and 16 in the efficacy study. Both studies were randomised and double blind. The primary outcome for the safety study was the occurrence of predefined serious adverse reactions over 6 months and for the efficacy study the reduction of corneal cystine crystal score (CCCS) by 1.00 or more units on photographs graded by a reading centre using a standardised protocol.
Results: No study subject developed any serious adverse reactions. In the efficacy study, 47% of eyes receiving the standard formulation experienced a reduction in the CCCS of ≥1.00 after 1 year, while 7% of eyes on the new formulation experienced such a decrease (p=0.04).
Conclusion: Although no serious adverse reactions were observed with either formulation, the new formulation was not as effective as the standard formulation.
Keywords: cysteamine, cystinosis, corneal crystals
Nephropathic cystinosis is an autosomal recessive lysosomal storage disorder caused by deficiency of the normal carrier mediated system that transports cystine out of lysosomes.1–4 Cystine crystals accumulate in the lysosomes of most cells and tissues. In the eye corneal cystine crystals result in severe photophobia, blepharospasm, and recurrent corneal erosions.5,6
The mainstay of treatment for cystinosis, oral cysteamine, if begun early and provided diligently, stabilises glomerular function, improves growth velocity, and obviates the requirement for thyroid hormone replacement.7–10 However, it does not dissolve corneal crystals,11 most probably because of inadequate local cysteamine concentrations.
In single centre trials, topical cysteamine treatment has been proved to efficiently dissolve corneal crystals and significantly alleviate the symptoms of photophobia, blepharospasm and eye pain that compromise the quality of life of cystinosis study subjects.12–14 The drops may be used at room temperature for up to 1 week. However, at room temperature, cysteamine oxidises to its disulfide form, cystamine. A randomised, controlled, double masked trial has eliminated cystamine as an alternative to cysteamine.15
The objective of this study was to evaluate the safety and efficacy of a new formulation, which retains cysteamine as a free thiol in stable form for 7 months at room temperature and up to 24 months under refrigeration.
METHODS
Study design
The study was designed as two prospective, double masked, randomised trials. The safety study was conducted at the National Eye Institute. The efficacy study was concurrently conducted at the National Eye Institute, University of Michigan School of Medicine and University of California, San Diego, School of Medicine. The protocol was approved by all participating centres’ institutional review boards.
Study subjects
Patients with cystinosis over 1 year of age, already receiving the standard topical cysteamine formulation, were eligible for the safety study. Exclusion criteria were a history of non-compliance with eye drops or the follow up schedule. For the efficacy study, patients with cystinosis 2–12 years of age who had never received topical cysteamine and whose corneal cystine crystal score (CCCS) was ≥1.00, were eligible. Cystinosis was diagnosed by a leucocyte cystine content above 2.0 nmol half cystine/mg protein (normal <0.2 nmol half cystine/mg protein) or the presence of corneal crystals in combination with a typical clinical course. Written informed consent was obtained from all study subjects or their parents.
Treatment
Study subjects were randomised to receive the new formulation in one eye and the standard formulation in the other eye. The standard cysteamine formulation was prepared by the NIH Pharmaceutical Development Service under Investigational New Drug (IND) No 40593 and is a 0.55% (50 mM) cysteamine hydrochloride solution with benzalkonium chloride 0.01%. The new formulation was obtained from Sigma-Tau Pharmaceuticals, under an amendment to IND No 40593 and is a 0.55% cysteamine hydrochloride solution with monosodium phosphate 1.85%, disodium EDTA 0.10%, and benzalkonium chloride 0.01%. Bottles of each formulation were labelled with a study subject number and right eye or left eye according to the random treatment assignment to maintain the treatment assignment masking of the study subjects, the clinic staff, and the photograph graders. To ensure masking, both medications were kept frozen, thawed over several hours before instillation, and were ordered to be given one drop in each eye, every waking hour. After thawing, bottles were allowed to be kept at room temperature for 1 week, with new bottles thawed weekly.
Conduct of the study
Ophthalmic evaluations performed at baseline consisted of best corrected visual acuity based on ETDRS charts or picture optotype visual acuity cards, slit lamp biomicroscopy, and photography by certified photographers following a standard protocol. Photographs were graded centrally at the National Eye Institute by two masked, independent graders, and were assigned a “corneal cystine crystal score” (CCCS) ranging from “0” (clarity at the centre) to “3.00” (greatest recognisable crystal density) in 0.25 increments.14 If the graders disagreed, a third grader assessed the photograph. The median score was used as the CCCS for that study subject.
Follow up ophthalmic examinations were performed at 6 months for the safety study subjects and every 3 months for 1 year for the efficacy study subjects. Study subjects were evaluated for the presence of photophobia and blepharospasm with the examination including best corrected visual acuity, slit lamp biomicroscopy, and slit lamp photography with assignment of a follow up CCCS by the masked graders.
The study subject or parent was asked to keep a daily calendar recording each administration of study medication and the study subject’s ocular status regarding side effects (namely, changes in vision, blurring, redness, episodes of acute corneal pain, other pain, irritation, itching) in each eye. These calendars were compiled by the study coordinator to assess compliance and adverse events after 1, 2, and 4 weeks, then every 3 months thereafter until month 12. The study coordinator scored compliance using one of “1” (poor, <4/day), “2” (good, 4–8/day), or “3” (excellent, >8/day). Average overall compliance was calculated based on these seven assessments. Telephone contact with study subjects was made 1 week, 2 weeks, and 1 month after initiation of study therapy to verify possible adverse reactions.
Primary outcome definitions
For the safety study the primary outcome was serious adverse reaction associated with the new formulation of cysteamine compared to the standard formulation. These serious adverse reactions included severe redness of 50% of the conjunctival surface related to administration of eye drops that did not blanch with 1:1000 topical epinephrine (adrenaline), persistent pain interfering with activities of daily living, or decrease in visual acuity from corneal damage greater than one line (more than five letters) on the ETDRS chart. In addition, blurring, redness, pain, irritation, and itching lasting more than 1 hour were evaluated in each eye and a comparison made between the eyes receiving the standard formulation versus the new formulation at 6 months.
For the efficacy study the proportion of study subjects with a reduction in CCCS of 1.00 unit or more in the eye treated with the new formulation and in the eye treated with the standard formulation was calculated.
Statistical analysis
Results of this study were primarily categorical, yielding several contingency tables. All results are analysed according to the study subject’s original treatment assignment (intention to treat). Several analysis techniques were used to test for significance, including McNemar’s test for paired observations, Fisher’s exact test for 2 × 2 tables, Wilcoxon signed rank and Cochran-Mantel-Haenszel test statistics.
RESULTS
Baseline evaluation
A total of 20 subjects were enrolled in the safety study and 16 in the efficacy study between September 1998 and July 1999. There were 13 (65%) males in the safety study and seven (47%) males in the efficacy study. All study subjects (100%) were white. Mean age at enrolment was 12.9 years (median 11.5 years, age range 5–27 years) in the safety and 6 years (median 6 years, age range 2–11 years) in the efficacy study. All eyes had a baseline CCCS of at least 1.25 in the efficacy study. One study subject prematurely discontinued therapy and did not return for follow up in each study.
Study outcome
None of the study subjects experienced any of the serious ophthalmic adverse reactions defined in the protocol and described in the Methods section. Only one study subject in the safety study lost more than one line of vision. This loss was attributed to testing inconsistency. Table 1 lists the number of eyes that experienced redness, itching, irritation, persistent pain, blurring or burning for more than 1 hour as well as events lasting less than 1 hour. There was no difference (p>0.5) between the treatments in the number of eyes with adverse events persisting longer than 1 hour. Stinging and burning sensations were the most common short term reactions and were more frequently reported in the eye treated with the new formulation in both studies but the difference was significant only in the safety study. Four of the safety and seven of the efficacy study subjects reported systemic adverse events that were thought to be unrelated to the study medications.
Table 1.
Ocular adverse events (combined safety and efficacy arms)
| Standard formulation | New formulation | |||
| Efficacy (n=15) | Safety (n=20) | Efficacy (n=15) | Safety (n=20) | |
| Events persisting in excess of 1 hour | ||||
| Redness | 3 | 4 | 3 | 6 |
| Itching | 1 | 0 | 1 | 1 |
| Irritation | 1 | 0 | 1 | 2 |
| Pain | 0 | 0 | 0 | 0 |
| Blurring | 0 | 0 | 0 | 0 |
| Burning | 1 | 0 | 1 | 0 |
| Discomfort | 2 | 0 | 2 | 0 |
| Severe pain | 0 | 0 | 1 | 0 |
| Total eyes with at least one event | 4 (27%) | 4 (20%) | 4 (27%) | 6 (30%) |
| Number of eyes with any event persisting less than 1 hour | 7 (47%) | 5 (25%) | 10 (67%) | 15 (75%) |
| Total eyes with at least one event of any duration | 8 (53%) | 6 (30%) | 11 (73%) | 15 (75%) |
In the safety study a decrease of 1.0 or greater (improvement) in CCCS after 6 months of therapy was observed for none out of nine eyes (0%) with baseline CCCS of ≥1.0 on the new formulation and three out of 10 eyes (30%) assigned to the standard formulation. Table 2 summarises changes in CCCS for all efficacy study subjects for the standard and the new formulation. In the efficacy study seven of the eyes receiving the standard formulation (47%) showed an improvement of one unit or more in the CCCS at 1 year, compared to one of the eyes (7%) receiving the new formulation (p=0.04 by Fisher’s exact test). Considering eyes as paired observations, one study subject showed a one unit or more improvement in both eyes, while six study subjects improved in only the eye receiving the standard formulation, and no study subjects improved in only the eye receiving the new formulation (p=0.031 by McNemar’s test for paired observations). Including study subjects with less than a one unit change, a Cochran-Mantel-Haenszel statistic test indicates that the means are significantly different (p=0.003). The median change from baseline to 1 year in CCCS is –0.75 for the standard formulation and 0.0 for the new formulation (p=0.0005 Wilcoxon signed rank).
Table 2.
Efficacy study at 1 year: corneal cystine crystal score (CCCS) changes from baseline
| Standard formulation | New formulation |
|
| ≥1 unit improvement* | 7 (47%) | 1 (7%) |
| <1 unit improvement | 4 (27%) | 2 (13%) |
| No change | 3 (20%) | 7 (47%) |
| <1 unit worsening | 1 (7%) | 5 (33%) |
| ≥1 unit worsening | 0 (0%) | 0 (0%) |
| Total | 15 | 15 |
*p=0.04.
Compliance scores indicated good to excellent compliance for 18 of the 19 safety study subjects and for all 15 efficacy study subjects with follow up.
DISCUSSION
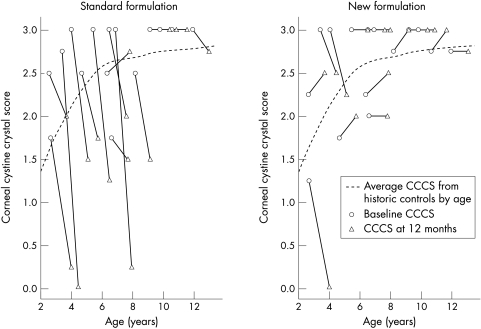
In the present study we tested the safety and efficacy of a new formulation that retains cysteamine as a free thiol at room temperature for approximately 7 months. The new formulation does not appear to be as effective in dissolving cornea crystals as the standard formulation. Figure 1 shows the baseline and follow up CCCS for each eye of study subjects enrolled in the efficacy study by age at enrolment and treatment overlaid on the loess curve approximating the CCCS of a historic control cohort previously published.14 After 12 months of treatment, eyes receiving the standard formulation fall well below the average CCCS score of untreated age matched historic controls while eyes receiving the new formulation have 12 month scores more similar to the untreated age matched historic cohort.
Figure 1.
Baseline and follow up corneal cystine crystal score (CSSS) by age and treatment of patients enrolled in efficacy study versus average CCCS for historic controls.14
Study subjects tolerated the standard formulation better than the new formulation, and neither formulation resulted in serious ophthalmic complications. Owing to the limited study subject population the study was unable to recruit 30 subjects and a sample size of 20 study subjects can not rule out event rates as great as 16% even if no events are observed. Use of the new formulation was associated with stinging and redness at instillation more often than the standard cysteamine formulation in people with prior experience using the standard formulation. It is possible that the new eye drops were washed out of the eyes due to tearing associated with the immediate discomfort from the drop use, accounting for the decreased efficacy. It is also possible that the new formulation does not penetrate the corneal stroma as effectively as the standard formulation, although a biological basis for this hypothesis is not available.
The results from this study underscore the importance of testing new treatments against the standard formulation, even when such treatments have pharmacologically equivalent amounts or concentrations of the active ingredient. Although the new formulation was not shown to be as effective as the standard formulation, this study represents the first multicentre, randomised and masked clinical trial to demonstrate that the standard formulation of cysteamine eye drops reduces corneal cystine crystals in cystinosis study subjects. Further work will be needed to develop an alternative treatment for corneal cystine crystals that does not have restrictive storage requirements.
Acknowledgments
The authors acknowledge the help of Lessie McCain for the excellent clinical coordination of the study; Ernest Kuehl, Leanne Ayres, and Marilois Chicca for grading the cornea photographs and assigning the cornea cystine crystal score (CCCS); Thomas M Clark, photographer, at the University of California, San Diego School of Medicine and Csaba Martonyi, photographer, at the University of Michigan. Supported in part by Sigma Tau Pharmaceuticals, Inc, contract N01-EY-6-2112, Clinical Trials and Statistical Study Monitoring and Coordination from the National Eye Institute, National Institutes of Health, and a grant from the General Clinical Research Centers Program, MO1 RR00827, of the National Center for Research Resources, National Institutes of Health.
REFERENCES
- 1.Gahl WA, Bashan N, Tietze F, et al. Cystine transport is defective in isolated leukocyte lysosomes from patients with cystinosis. Science 1982;217:1263–5. [DOI] [PubMed] [Google Scholar]
- 2.Gahl WA, Tietze F, Bashan N, et al. Defective cystine exodus from isolated lysosome-rich fractions of cystinotic leucocytes. J Biol Chem 1982;257:9570–5. [PubMed] [Google Scholar]
- 3.Jonas AJ, Smith ML, Schneider JA. ATP-dependent lysosomal cystine efflux is defective in cystinosis. J Biol Chem 1982;257:13185–8. [PubMed] [Google Scholar]
- 4.Town M, Jean G, Cherqui S, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet 1998;18:319–24. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser-Kupfer MI, Caruso RC, Minckler DS, et al. Long-term ocular manifestations in nephropathic cystinosis. Arch Ophthalmol 1986;104:706–11. [DOI] [PubMed] [Google Scholar]
- 6.Gahl WA, Kaiser-Kupfer MI. Complications of nephropathic cystinosis after renal failure. Pediatr Nephrol 1987;1:260–8. [DOI] [PubMed] [Google Scholar]
- 7.Gahl WA, Reed GF, Thoene JG, et al. Cysteamine therapy for children with nephropathic cystinosis. N Engl J Med 1987;316:971–7. [DOI] [PubMed] [Google Scholar]
- 8.Markello TC, Bernardini IM, Gahl WA. Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med 1993;328:1157–62. [DOI] [PubMed] [Google Scholar]
- 9.Gahl WA, Charnas L, Markello TC, et al. Parenchymal organ cystine depletion with long-term cysteamine therapy. Biochem Med Metab Biol 1992;48:275–85. [DOI] [PubMed] [Google Scholar]
- 10.Kimonis VE, Troendle J, Rose SR, et al. Effects of early cysteamine therapy on thyroid function and growth in nephropathic cystinosis. J Clin Endocrinol Metab 1995;80:3257–61. [DOI] [PubMed] [Google Scholar]
- 11.Cantani A, Giardini O, Ciarnella Cantani A. Nephropathic cystinosis: ineffectiveness of cysteamine therapy for ocular changes. Am J Ophthalmol 1983;95:713–4. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser-Kupfer MI, Fujikawa L, Kuwabara T, et al. Removal of corneal crystals by topical cysteamine in nephropathic cystinosis. N Engl J Med 1987;316:775–9. [DOI] [PubMed] [Google Scholar]
- 13.Kaiser-Kupfer MI, Gazzo MA, Datiles MB, et al. A randomized placebo-controlled trial of cysteamine eye drops in nephropathic cystinosis. Arch Ophthalmol 1990;108:689–93. [DOI] [PubMed] [Google Scholar]
- 14.Gahl WA, Kuehl EM, Iwata F, et al. Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab 2000;71:100–20. [DOI] [PubMed] [Google Scholar]
- 15.Iwata F, Kuehl EM, Reed GF, et al. A randomized clinical trial of topical cysteamine disulfide (cystamine) versus free thiol (cysteamine) in the treatment of corneal cystine crystals in cystinosis. Mol Genet Metab 1998;64:237–42. [DOI] [PubMed] [Google Scholar]