Abstract
The Agouti-related protein (AgRP) is a central orexigenic peptide leading to increased food intake when ubiquitously overexpressed. AgRP-deficient (AgRP−/−) mice have either no phenotype or present an age-related leanness. In this study, AgRP−/− mice were fed alternate high fat or low fat diets in an effort to determine whether AgRP is a mediating factor for the effects of dietary fat on metabolic parameters. There were no striking metabolic differences between AgRP−/− and the equally obese wild type littermates but AgRP−/− mice displayed a significantly longer life span. The point estimate of median survival for the AgRP−/− group was 9.8% greater while the significantly low hazard ratio (0.494) suggests that mortality incidence of AgRP−/− mice is less than one-half that of the wild type reference population. It is concluded that although AgRP−/− mice become morbidly obese consuming a high fat diet (a landmark feature for a shortened life span), they seem to overcome obesity- and age-related patholologies and live significantly longer than their metabolically similar wild type littermates.
Keywords: Body weight, longevity, diet, metabolism, neuropeptide
INTRODUCTION
The Agouti-related protein (AgRP) plays an important role in the regulation of food intake and energy balance [1]. Hypothalamic AgRP is elevated in obese and diabetic mice [2] while AgRP transgenic mice are hyperphagic and obese [3]. AgRP acts as an endogenous antagonist [4] or inverse agonist [5] blocking α-MSH signaling at the melanocortin receptors 3 and 4. AgRP/NPY-expressing neurons in the hypothalamus appear to be essential for controlling energy homeostasis [6-8] but studies with AgRP-deficient mice have raised questions about the functional properties of this gene [9]. Specifically, earlier studies did not identify a phenotype for AgRP-deficient mice [10] but a recent report showed that AgRP−/− mice presented an age-dependent lean phenotype, increased body temperature, and increased metabolic rate [11]. Studies in humans have shown that single nucleotide polymorphisms (SNPs) impairing AgRP expression or protein function are associated with reduced BMI, lower incidence of type 2 diabetes, prevention of late-onset obesity, and macronutrient selection [12-14].
The present manuscript describes observations on a line of AgRP-deficient mice that were placed on alternate high fat (HF) or low fat (LF) diets in an effort to extricate an effect of AgRP-deficiency on metabolic parameters in relation to dietary fat. The AgRP-deficient mice again did not show radical differences from wild type littermates with respect to body weight, energy expenditure, diurnal food intake, diurnal activity, and body composition. In contrast, AgRP-deficient mice displayed the novel phenotype of extended lifespan while maintained on a high fat (HF) diet during the second half of life. The effect of AgRP deficiency appears to resemble that of calorie restriction while ameliorating to a degree the effects of long-term HF feeding on lifespan [15, 16]. These data are discussed in relation to metabolic similarities between AgRP−/− mice and their wild type littermates that experienced the anticipated shorter life span under the same nutritional paradigm.
MATERIALS AND METHODS
Mice
AgRP−/− mice were the kind gift of Dr. Qian (Department of Obesity and Metabolic Research, Merck Research Laboratories, Rahway, NJ 07065). Mice were subsequently backcrossed for three generations to C57BL/6J wild type mice and bred at the Pennington Biomedical Research Center (PBRC). The genetic background of the mice is therefore considered as “mixed”, with 87.5% C57B6 and 12.5% 129sv. Mice were genotyped by PCR using a forward primer on the neo cassette and a reverse primer on AgRP genomic DNA. A total of 14 wild type (WT) males, 16 AgRP−/− males, 4 WT females, 7 AgRP−/− females were used for the experiments. The study protocol was approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee. Mice were fed regular chow diet (12.1 % fat, mouse Labdiet #5001, Purina Mills, St. Louis, MO) at weaning and kept on a 12/12h dark/light cycle (lights on 6.00 and off 18.00). High fat (60 kcal%, D12492) and low fat (10 kcal%, D12450B) diets were commercially purchased (Research Diets, New Brunswick, NJ).
Diets and study design
Mice consumed alternate diets as follows: at 3 weeks of age chow; at 16 weeks HF diet; at 21 weeks LF diet; at 41 weeks chow; at week 42 HF diet until death. Different diets were used in order to tease out potential effects of dietary fat on metabolic parameters of AgRP−/− mice. As such, this study was not originally designed to be a longevity study per se.
Metabolic measurements
Oxygen consumption (converted to energy expenditure), diurnal food intake, and diurnal activity were measured in metabolic chambers (Columbus Instruments, Columbus, OH).
Body composition
Fat, free body fluid, and lean tissue values were measured in a Nuclear Magnetic Resonance instrument (Bruker Optics Inc, The Woodlands, TX). Percent body fat and muscle mass were calculated by dividing with total mass.
Cold exposure
A subset of 4 wild type and 6 AgRP−/− female mice was used to measure rectal temperature by insertion of a thermometer probe. Mice were placed in a 4 °C incubator and temperature was measured at the beginning of the experiment and every one hour thereafter for the duration of six hours. Upon completion of the experiment mice were returned to their original cages where they fully recovered.
Statistical Methods / Survival
For each genotype a nonparametric estimate of survival, recorded in weeks from birth, was computed by means of the product-limit estimator. Results are presented graphically in Fig 3. A stratified log-rank test for genotype, controlling for the effect of sex, was employed to test equality of survival across genotype groups. Survival of the AgRP−/− group was found to exceed that of their counterparts (p=0.045). And, to further evaluate the role of both the AgRP genotype and gender as determinants of survival, a (Cox) proportional hazards model for these data was evaluated. While there was no evidence of a gender effect in this model (p=0.83), the estimated hazard ratio of 0.494 for the KO genotype, relative to wild type, was statistically significant (p=0.049). SAS Software version 9.1.3 for Windows (SAS Institute, Cary, NC) was used to implement the various survival models. Statistical significance is reported for all tests relative to a 5% type1 error rate.
Figure 3.
Survival function estimates of AgRP-deficient (−/−) and wild type (+/+) littermate mice (males and females pooled together per genotype) (the asterisk “*” represents one censured observation corresponding to a surviving AgRP−/− male.
Differences in body weight and body composition between WT and AgRP−/− groups of mice were evaluated by the Student's T-test.
RESULTS AND DISCUSSION
Overexpression or intracerebroventricular administration of AgRP leads to hyperphagia and the development of obesity. An earlier publication surprisingly showed that AgRP-deficient mice did not present an anticipated phenotype such as increased metabolic rate or reduced food intake [10]. A more recent report however showed that AgRP−/− mice exhibit increased metabolic rate and increased body temperature but in an age-dependent fashion [11]. The mice described here were derived from those reported earlier [10]. Mice were backcrossed three times to C57BL/6J and homozygous AgRP−/−were compared to WT littermates. Mice were placed on alternate HF and LF diets in order to examine whether dietary fat content could reveal a metabolic phenotype (e.g. leanness) in this AgRP knockout strain.
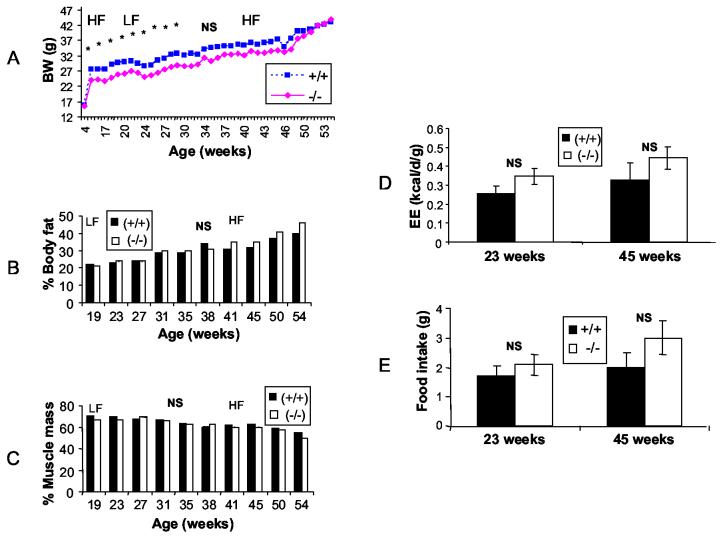
The body weight of male AgRP−/− did not differ significantly from that of the WT littermates and, in contrast to expectations, AgRP−/− males weighed more but in an age dependent fashion (35 weeks of age onwards but not always at statistically significant levels – FIGURE 1). A heavier phenotype by the male AgRP−/− was also reported earlier [10]. Muscle mass was higher while fat mass was lower in the male AgRP−/− mice but again not always at statistically significant levels. The higher muscle mass could perhaps explain the marginally higher body weight of these mice. There were no significant differences between AgRP−/− and WT littermates in any other metabolic parameter measured (24-h energy expenditure and diurnal food intake).
Figure 1.
Metabolic characteristics of AgRP-deficient (−/−) and wild type littermate (+/+) male mice: (A) Body weight, (B) Percent body fat, (C) Percent muscle mass, (D) Energy expenditure, (E) Food intake.
The female AgRP−/− mice exhibited a leaner phenotype from WT littermates (FIGURE 2) but it was statistically significant only between the ages of 12 to 30 weeks, a period during which all mice consumed LF diet. Female AgRP−/− mice had higher energy expenditure and seemed to be eating more food than WT littermates (which is inconsistent with the anticipated effect of AgRP-deficiency) but not at statistically significant levels (FIGURE 2). Of interest was an apparent cold-sensitivity by the AgRP−/−females (P<0.05) when placed at 4 °C but after 4 hours they recovered sufficiently to match the WT littermates (data not shown).
Figure 2.
Metabolic characteristics of AgRP-deficient (−/−) and wild type littermate (+/+) female mice: (A) Body weight, (B) Percent body fat, (C) Percent muscle mass, (D) Energy expenditure, (E) Food intake.
The most striking phenotype of the AgRP−/− mice was their extended life span. Survival of the AgRP−/− mice was found to differ significantly from that of the wild type population, based on a stratified log-rank test (p=0.045). The point estimate of median survival for the AgRP−/− mice (101 weeks) exceeded that of their counterparts by 9.8%. Furthermore, the estimated hazard ratio of 0.494 for the AgRP−/− animals relative to wild type indicates that AgRP-deficiency is associated with an approximately 2-fold reduction in mortality incidence. These findings imply a considerable extension to life span associated with AgRP-deficiency, especially considering that these mice consumed a mandatory HF diet from 7.7 months of age and up to their death. Moreover, these mice gained significant amount of weight (reaching up to 78 g) that did not differ from the weight gained by WT littermates. It is noteworthy that the longest lived AgRP−/− mouse was a male that reached 2 years and 9 months of age. This mouse weighed from 55 to 68 g between the ages of 12-24 months with its weight gradually declining to 39.5 g by the age of 2yr 7mo old.
Life spans of mouse strains are known to differ. C57BL/6J mice, in general, live longer than A/J, BALB/cJ, and DBA/2J mice [17] but mutations and obesity in mice of C57BL/6J genetic background shorten the life span [18]. Wild strain alleles tend to extend maximum life spans [19] while big mice die young with early life body weight being a predictor of longevity [20]. Gerontological data show that C57BL/6J males and females live on average 878 and 794 days respectively while consuming regular chow [21]. The mice presented here were of a mixed genetic background (a theoretical 87.5% of C57BL/6J genotype) living on average 707 days for the AgRP−/− and 644 days for the WT mice, consuming an HF diet from the age of 7.7 months. It is unlikely that the genetic background itself played a significant role to the differences in lifespan between the AgRP−/− and WT mice because they were all littermates. Similarly, there were no striking metabolic differences between AgRP-deficient and WT mice and therefore it is likely that a different type of a factor may be responsible for the extended life span of the AgRP−/− mice. The growth hormone and insulin growth factor have been shown to play significant roles in the aging process [22, 23] but neither was assessed in the AgRP−/− mice since the prolonged life span was an unexpected phenotype.
In humans, lower adiposity and caloric restriction leads to longevity [16], while obesity is a significant factor contributing to a decreased lifespan [15]. Carriers of the Ala67Thr SNP in AgRP are less likely to develop visceral obesity in later age [13] possibly due to macronutrient selection [24], which suggests that AgRP genetic defects may provide a mechanism for protecting humans against late onset visceral obesity. The data presented here show that AgRP-deficient mice develop an advantage that enables them to overcome the negative effects of morbid obesity and live significantly longer than their wild type littermates. These data also suggest that the involvement of AgRP in the process of aging may be independent from its role in feeding behavior. This study was not originally designed to be a longevity study and therefore additional experiments using larger sample sizes would need to be conducted to confirm the findings presented here.
Table 1.
Summary of mice, genotypes, gender, and statistical analyses of survival curves.
| Genotype | N | Events | Median survival (95% CL) | χ2 Log-Rank | Hazard Ratio |
|---|---|---|---|---|---|
| WT | 16 (12M, 4F) |
100% (16) | 92 (75-97) | 4.02 | 0.494 |
| P-value: | P-value: | ||||
| AgRP−/− | 21 (14M, 7F) |
100% (21) | 101 (88-108) | 0.045* | 0.049* |
significant at P<0.05
ACKNOWLEDGMENTS
This work was supported in part by NIH grant DK62156 to GA. We thank Dr. Qian (Department of Obesity and Metabolic Research, Merck Research Laboratories, Rahway, NJ 07065) for kindly making available the original AgRP−/− mice. We also thank Mss. Chantal Charbonneau, Tammy Fairburn, and Adrienne Cain for excellent technical assistance. We express our appreciation to Dr. Barry Roberts, DVM, Ph.D., and the staff of Comparative Biology at the Pennington Biomedical Research Center for their excellent veterinary care of the mice.
REFERENCES
- 1.Stutz AM, Morrison CD, Argyropoulos G. The Agouti-related protein and its role in energy homeostasis. Peptides. 2005;26:1771–1781. doi: 10.1016/j.peptides.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up- regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 3.Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL. Overexpression of Agrt leads to obesity in transgenic mice. Nat Genet. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- 4.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 5.Haskell-Luevano C, Monck EK. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul Pept. 2001;99:1–7. doi: 10.1016/s0167-0115(01)00234-8. [DOI] [PubMed] [Google Scholar]
- 6.Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, Bloom SR. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. Faseb J. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- 7.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Bruning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 8.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 9.Flier JS. AgRP in energy balance: Will the real AgRP please stand up? Cell Metab. 2006;3:83–85. doi: 10.1016/j.cmet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, Chen A, Camacho RE, Shearman LP, Gopal-Truter S, MacNeil DJ, Van Der Ploeg LH, Marsh DJ. Neither Agouti-Related Protein nor Neuropeptide Y Is Critically Required for the Regulation of Energy Homeostasis in Mice. Mol Cell Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, Yancopoulos GD, Wiegand SJ, Sleeman MW. Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab. 2005;2:421–427. doi: 10.1016/j.cmet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Argyropoulos G, Rankinen T, Bai F, Rice T, Province M, Leon A, Skinner J, Wilmore J, Rao D, Bouchard B. The agouti related protein and body fatness in humans. International Journal of Obesity. 2003;27:276–280. doi: 10.1038/sj.ijo.802201. [DOI] [PubMed] [Google Scholar]
- 13.Argyropoulos G, Rankinen T, Neufeld DR, Rice T, Province MA, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. A polymorphism in the human agouti-related protein is associated with late-onset obesity. J Clin Endocrinol Metab. 2002;87:4198–4202. doi: 10.1210/jc.2002-011834. [DOI] [PubMed] [Google Scholar]
- 14.Bonilla C, Panguluri RK, Taliaferro-Smith L, Argyropoulos G, Chen G, Adeyemo AA, Amoah A, Owusu S, Acheampong J, Agyenim-Boateng K, Eghan BA, Oli J, Okafor G, Abbiyesuku F, Johnson T, Rufus T, Fasanmade O, Chen Y, Collins FS, Dunston GM, Rotimi C, Kittles RA. Agouti-related protein promoter variant associated with leanness and decreased risk for diabetes in West Africans. Int. J. Obes. Relat. Metab. Disord. 2005 doi: 10.1038/sj.ijo.0803047. [DOI] [PubMed] [Google Scholar]
- 15.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 16.Wolf G. Calorie restriction increases life span: a molecular mechanism. Nutr Rev. 2006;64:89–92. doi: 10.1301/nr.2006.feb.89-92. [DOI] [PubMed] [Google Scholar]
- 17.Goodrick CL. Life-span and the inheritance of longevity of inbred mice. J Gerontol. 1975;30:257–263. doi: 10.1093/geronj/30.3.257. [DOI] [PubMed] [Google Scholar]
- 18.Goodrick CL. Body weight change over the life span and longevity for C57BL/6J mice and mutations which differ in maximal body weight. Gerontology. 1977;23:405–413. doi: 10.1159/000212216. [DOI] [PubMed] [Google Scholar]
- 19.Klebanov S, Astle CM, Roderick TH, Flurkey K, Archer JR, Chen J, Harrison DE. Maximum life spans in mice are extended by wild strain alleles. Exp Biol Med (Maywood) 2001;226:854–859. doi: 10.1177/153537020122600908. [DOI] [PubMed] [Google Scholar]
- 20.Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- 21.Kunstyr I, Leuenberger HG. Gerontological data of C57BL/6J mice. I. Sex differences in survival curves. Journal of Gerontology. 1975;30:157–162. doi: 10.1093/geronj/30.2.157. [DOI] [PubMed] [Google Scholar]
- 22.Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- 23.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. From the Cover: Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loos RJ, Rankinen T, Rice T, Rao DC, Leon AS, Skinner JS, Bouchard C, Argyropoulos G. Two ethnic-specific polymorphisms in the human Agouti-related protein gene are associated with macronutrient intake. Am J Clin Nutr. 2005;82:1097–1101. doi: 10.1093/ajcn/82.5.1097. [DOI] [PubMed] [Google Scholar]