Abstract
Aims: To report on the use of trypan blue (TB) 0.06% for staining the internal limiting membrane (ILM) and epiretinal membrane (ERM) during vitrectomy and report on their histology.
Method: 14 consecutive patients with idiopathic macular hole or macular pucker (seven patients each) were prospectively recruited for ILM or ERM peel respectively. After pars plana vitrectomy and induction of posterior vitreous detachment, 0.5 ml TB 0.06% in phosphate buffered saline (VisonBlue) was injected over the posterior pole in an air filled eye and left for 2 minutes. The stained tissue was peeled with intraocular forceps. Specimens were evaluated using histochemical and immunohistochemical methods.
Results: The average follow up was 4.4 months. Internal limiting membranes and epiretinal membranes were stained satisfactorily in all cases and removed successfully. Eight patients (57%) had improvement of 2 or more Snellen lines. All seven macular holes closed. In the ERM cases, no residual membranes were observed clinically, at the latest follow up. No complications relating to the use of the dye were encountered intraoperatively or postoperatively. Of the 14 procedures, nine (four macular hole and five macular pucker) yielded sufficient tissue for histopathological evaluation. Histological and immunohistological assessment revealed that the morphology of these specimens was similar to that observed in macular hole ILM and macular pucker ERM removed without the aid of dye.
Conclusion: TB staining facilitated the identification and delineation of ILM and ERM removal during the surgical management of macular holes and macular pucker. The visual outcome of this series and the specimens removed suggest they are no different from those without TB staining. Its use in posterior segment appears to be safe but further studies are required to investigate its long term safety.
Keywords: trypan blue, internal limiting membrane, epiretinal membrane, histopathology, macular hole
The peeling of internal limiting membrane (ILM) and epiretinal membrane (ERM) is a challenging manoeuvre in vitreoretinal surgery. Some of the technical difficulties usually encountered during ILM peel surgery are incising or grasping the ILM surface; finding the edge of the initial incision on the ILM; creating a flap; and, maybe most importantly, identifying the areas where the ILM has already been peeled. ERM may be difficult to visualise if fibrosis or pigment deposition is absent, and as a result the removal of the membrane may be incomplete. Inadvertent trauma to the retina may also occur, in particular during ILM peeling.
In the past few years, indocyanine green (ICG) has been widely adopted for the staining of ILM especially during macular hole surgery.1–5 Although in vitro studies showed ICG does not affect cellular activities,6 there has been growing clinical evidence that ICG might not be as safe as originally thought. Cases of ICG toxicity7 and its alteration of the surgical cleavage plane have been reported.3 For example, it has been postulated that elements of neuroretina are removed with ICG treated ILM.3 Conversely, proliferative vitreoretinopathy (PVR) ERM peeled with trypan blue (TB) were reported to lack ILM.8 Since PVR membranes typically include ILM, these two studies suggest that there is a difference in cleavage plane between ICG and TB stained retinal surface tissue. Some surgeons find ICG staining of the ILM indispensable and most agree there is a need for a safe and effective dye for staining the ILM to facilitate removal.1–5
TB is used to stain the anterior lens capsule during cataract surgery in a relatively low concentration and proved to be effective and safe.9 We evaluated the potential use of TB for the intraoperative staining the ILM and macular pucker membranes in order to facilitate their identification and complete excision. The visual outcome of these patients was evaluated and the nature of the excised tissue was confirmed with histopathology.
MATERIALS AND METHODS
Approval of the hospital ethics committee was obtained for the study and included permission for the collection and analysis of histological samples. Individuals over 16 years of age requiring vitrectomy surgery for epiretinal membrane removal or macular hole repair were prospectively recruited. Only patients undergoing the first operation for macular hole repair or macular pucker membrane peel were included.
Informed consent was obtained from each patient. Preoperative data collected included age, sex, best corrected Snellen visual acuity, underlying condition/staging. Postoperative best corrected Snellen visual acuity, anatomical outcome, and histopathological findings were recorded.
Ten patients were female and four were male. Seven eyes underwent surgery for macular hole. Five eyes were stage III macular holes and two were stage IV. Before surgery, epiretinal membranes associated with the macular hole were identified in none of the seven cases when the eyes were examined clinically using slit lamp biomicroscopy. Seven patients underwent surgery for macular pucker. The aetiologies of the macular pucker were previous retinal detachment (four), branch retinal vein occlusion (one), and idiopathic (two).
Commercially available TB 0.06% (VisonBlue, DORC International bv, Scheijndelveweg 2, 3214VN Zuidland, Netherlands) in phosphate buffered sodium chloride (NaCl) was used. TB has already been tested for biocompatibility and CE approval for intraocular use (cytotoxicity, extract, 24 hours, end point dilution test, conducted according to ISO 10993/EN 30993 standard by KEMA Medical, Netherlands).
Surgical technique
Fourteen consecutive patients with idiopathic macular hole or macular pucker underwent a three port pars plana vitrectomy, including where necessary the induction of a posterior vitreous detachment and a fluid/air exchange. A volume of 0.5 ml TB 0.06% solution was injected into the air filled vitreous cavity over the posterior pole. After 2 minutes, still under air, the dye was removed using a flute needle. An air/fluid exchange was then carried out. The ILM and the ERM was stained a faint blue colour and was clearly visually under standard illumination. The blue stained tissue was directly engaged with the intraocular forceps (Microforceps: Eckardt End Gripping Forceps, DORC International bv, Scheijndelveweg 2, 3214VN Zuidland, Netherlands) to create a flap and then peeled from the nerve fibre layer plane usually in a capsulorrhexis fashion.10 In the patients with macular hole, we injected 0.1 ml of platelets concentrate as per our normal routine for the treatment of macular holes. C3F8 gas mixture, 14%, was used and the patients were postured supine for 3 hours immediate postoperatively, followed by a prone position for 50 minutes in every waking hour for 2 weeks. In the patients with macular pucker, routine closure, and no tamponade agents were used.
Light microscopy and immunohistochemistry
Specimens were fixed in 10% neutral buffered formalin, dehydrated in graded concentrations of ethanol, and embedded in paraffin wax. Sections of wax embedded tissue were cut, dewaxed, and stained with haematoxylin and eosin. Further sections were stained with periodic acid Schiff (PAS) method. A record was made of the presence or absence of ILM and cells in the tissue.
Immunohistochemical methods were used to detect glial, retinal pigment epithelial (RPE) cell, or neural elements in the tissue.11 The antibodies employed were mouse monoclonal antibodies to glial fibrillary acidic protein (GFAP), a broad spectrum of cytokeratins and neurofilament protein (NFP) respectively (all from Dako, Ely, UK). Immunoreactive sites were visualised red (using 3-amino-9-ethylcarbazole or fast red). Controls were conducted as previously described.11 Sections were counterstained with Mayer’s haematoxylin and examined by bright field microscopy.
RESULTS
Clinical findings
Patient demographics, diagnosis, and outcome were tabulated in Table 1. Mean patient age was 60.7 (range 38–68). Preoperative best corrected Snellen visual acuity ranged from 1/60 to 6/6. Postoperative best corrected Snellen visual acuity ranged from 5/60 to 6/6. Vision was improved or maintained in all 14 (100%) patients. Eight patients (57%) had improvement of 2 or more Snellen line. The mean follow up period was 4.4 months (range 2–6 months). All seven macular hole cases had successful closure of the hole. At the latest follow up, when examined clinically using biomicroscopy, none of the seven ERM cases showed any evidence of residual membranes or recurrence. In all 14 eyes, the ILM or the ERM was adequately stained and visualised. After initiation of the flap, ILM and ERM were clearly seen and grasped with intraocular forceps. There was a distinct contrast between stained ILM/ERM and unstained retina thus, enabling and facilitating the complete removal of these tissue (Fig 1). Where possible, the ILM was removed up to both superior and inferior temporal arcades. No intraoperative or postoperative complications were observed. Attempts were made to retrieve the surgically excised tissue from each case. The harvesting of small ERM and vitreous specimens such as ILM is notoriously difficult.12 Using TB, however, we were successful in collecting nine out of 14 specimens, including four out of seven ILM, for histological evaluation (Table 2).
Table 1.
Patient demographics, diagnosis, and outcome
| Patient No | Sex/age | Preop VA | Condition/staging or aetiology | Postop VA/(follow up) (months) | Anatomical outcome |
| 1 | F 68 | 1/60 | MH/ IV | 6/24 (6) | Closed |
| 2 | M 47 | 6/36 | ERM/PVR | 6/36 (5) | Flat |
| 3 | F 77 | 6/18 | MH/ III | 6/12 (4) | Closed |
| 4 | M 49 | 6/6 | ERM/idiopathic | 6/6 (4) | Flat |
| 5 | F 58 | 6/60 | ERM/idiopathic | 6/18 (4) | Flat |
| 6 | F 68 | 6/36 | MH/ III | 6/24 (2) | Closed |
| 7 | F 67 | 6/36 | MH/ IV | 6/18 (2) | Closed |
| 8 | M 38 | 6/24 | MH/ III | 6/9 (2) | Closed |
| 9 | F 54 | 6/18 | ERM/PVR | 6/12 (2) | Flat |
| 10 | F 68 | 6/36 | MH/ III | 6/18 (4) | Closed |
| 11 | F 57 | 6/12 | ERM/PVR | 6/6 (2) | Flat |
| 12 | F 58 | 3/60 | ERM/PVR | 6/24 (2) | Flat |
| 13 | F 73 | 2/60 | ERM/BRVO | 6/18 (2) | Flat |
| 14 | M 68 | 5/60 | MH/ III | 5/60 (2) | Closed |
VA = best corrected Snellen visual acuity, Staging = as per Gass classification,19 MH = macular hole, ERM = epiretinal membrane.
Figure 1.
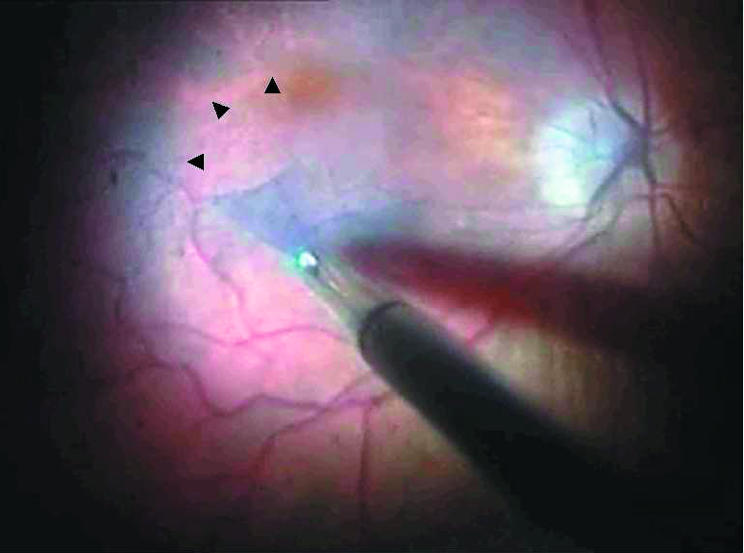
Operating microscope view demonstrating the continuous ILM peel in progress. A triangular flap of ILM is seen being peeled by forceps. Arrowheads indicate the area of ILM peeled in contrast with the unpeeled trypan blue stained ILM
Table 2.
Histopathological findings in the excised epimacular specimens
| Patient No | Clinical diagnosis | ILM | ERM morphology | GFAP | NFP | Cytokeratins† |
| 14 | MH | + | M | + | + | − |
| 6 | MH | + | − | NA | NA | NA |
| 7 | MH | + | − | NA | NA | NA |
| 8 | MH | + | − | NA | NA | NA |
| 2* | ERM | + | F/C | + | + | − |
| 4 | ERM | + | F/C | + | − | + |
| 5 | ERM | + | F/C | + | + | − |
| 13 | ERM | + | M | + | − | − |
| 12 | ERM | ? | F/C | NA | NA | NA |
MH = macular hole, ILM = internal limiting membrane, ERM = epiretinal membrane, GFAP = glial fibrillary acidic protein (a marker of glial cells), NFP = neural fibrillary protein (a marker of nerve cells), M = cellular monolayer, F/C = fibrocellular, ? = uncertain, NA = not available.
*Fragment of neural retina present in specimen; †A marker of retinal pigment epithelial cells.
Histopathological findings in macular hole ILM specimens
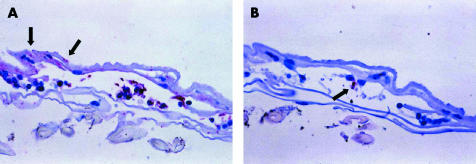
ILM was observed in all four of the macular hole specimens that were examined microscopically (Table 2). One of these specimens (from patient No 14) also contained an unexpected cellular component. These cells were on the smooth (vitreous) surface of the ILM (that is, they constituted an ERM) and immunohistochemistry revealed that they were glial in origin (Table 2, Fig 2). A NFP immunoreactive focus was noted in this ERM (Table 2, Fig 2) but no RPE cells were detected in the specimen. Glial elements were seen on the retinal (corrugated) side of the ILM, but neural elements were not apparent on this side of the ILM (Fig 2). There was insufficient tissue in the remaining macular hole specimens for immunohistochemistry.
Figure 2.
Photomicrographs of the tissue removed from a macular hole case (Patient No 14). Sections have been stained by immunohistochemistry for glial (A) or neural (B) elements (red chromogen, haematoxylin counterstain). In (A), glial cells (stained red) can be seen on the smooth surface of the folded ILM. There are also foci of glial immunoreactivity on the corrugated (retinal) side of the ILM (arrows). In (B), a neural element (arrow) is seen in the epiretinal tissue, but no neural component is seen on the retinal side of the ILM (original magnification ×600).
Histopathological findings in macular pucker ERM specimens
Four of the five ERM retrieved for histology contained ILM and all five also included a cellular or fibrocellular component (Table 2). In one specimen (from patient No 2), there was also a distinct fragment of neural retina (Fig 3).
Figure 3.
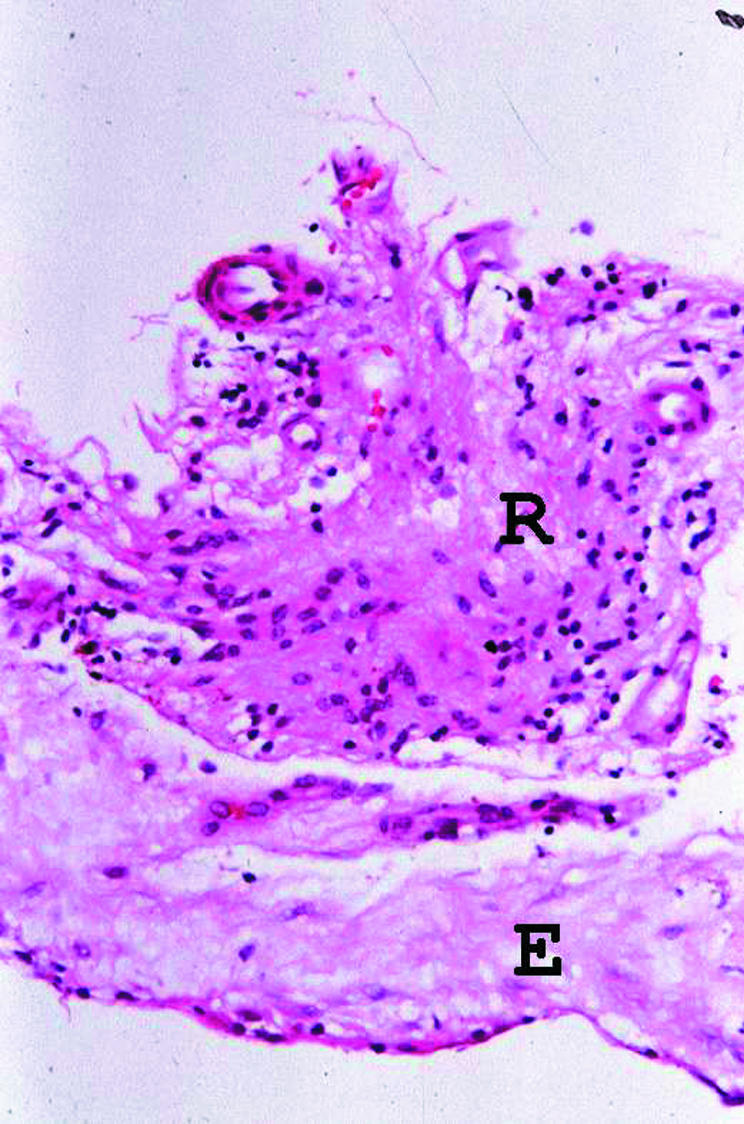
Photomicrograph of the ERM specimen from patient No 2, showing a fragment of retinal tissue (R) to which the fibrocellular ERM (E) is adherent (haematoxylin and eosin, original magnification ×300).
In four of the five ERM cases there was sufficient tissue to undertake immunohistochemical assessment and all of these specimens contained glial elements. In addition, two of the ERM (from patient No 2 and 5) contained neural elements (Table 2). RPE cells were detected in one of the specimens (from patient No 4). Glial and neural elements on the retinal side of the ILM was observed in the retinal fragment from patient No 2 and glial elements were also noted on the retinal side of ILM in two other specimens (from patients 5 and 13).
DISCUSSION
In cataract surgery, staining of the anterior lens capsule with a vital dye was found to be extremely useful in visualisation of the anterior capsule to facilitate capsulorhexis. Similarly, the dye could be used as an aid to stain internal limiting membranes and epiretinal membranes in posterior segment surgery.
ICG has been shown to be helpful in staining ILM and thus facilitating its removal.1–5 Although it has been used in human for many years and shown to be not toxic, there is growing evidence that this might not entirely be the case. Gandorfer et al3 suggested that the cleavage plane of ILM with ICG staining might be more retinal than expected. Histopathology from their series showed plasma membranes of Muller cells on the retinal surface of the ILM. Weinberger et al13 reported persistent ICG fluorescence 6 weeks after ILM peel with the use of ICG in a case of macular hole surgery. All these factors might limit the potential of visual recovery if ICG is used to stain ILM.
TB is mainly used in anterior segment surgery to stain human corneal tissue for identification of devitalised endothelial cells14 and in staining anterior capsule in cataract surgery.9 It has recently been found that it might have a role in posterior segment surgery as well. Feron et al8 reported the usefulness of TB in aiding removal of proliferative vitreoretinopathy (PVR) membranes.
The morphology of the macular hole ILM and macular pucker ERM specimens removed with TB is generally similar to that of macular hole and macular pucker membranes removed without the aid of a dye.15 For example, as has been observed in membranes removed without the aid of dye, ERM associated the PVR, idiopathic macular pucker and macular hole generally contained ILM and glial cells. The observation of ILM in the TB stained PVR membranes differs from a report by Feron and colleagues, who did not find ILM in the specimens of ERM peeled with TB staining in their cases of PVR.8
In addition to ILM, a fragment of neural retina was observed in one ERM. Moreover, glial elements were found on the apparent retinal side of the ILM in two other macular pucker membranes and in the macular hole specimen with a clinically silent ERM. The presence of glial and/or neural components on the retinal side of the ILM would suggest retinal damage and is consistent with the findings of a recent electron microscopic ILM study by Gandorfer and coworkers.3 However, Gandorfer and associates3 attributed their findings to the use of ICG whereas our observations suggest that, at least where ERM are present, the dye is not responsible for the presence of retinal elements on the ILM. Indeed, similar fragments may also be seen in ERM specimens removed without any dye.15 The use of dye may or may not influence the surgical cleavage plane which may be also be influenced by surgical technique.16
Conversely, in three specimens (one macular hole specimen and two of the ERM) neural elements appeared within the epiretinal tissue. The presence of neural tissue on the vitreous side of the ILM is interesting. This finding might be explained by recent work by Sethi et al17 which suggests that neural elements found in PVR membranes may reflect outgrowth from the retina into the developing membrane.
Our choice of 0.06% TB is supported by the possible toxicity to photoreceptors in higher concentration in animal studies.18 From our experience, TB stained both ILM and ERM adequately allowing complete removal. ICG has been shown to stain ILM selectively2 and with increasing evidence of its toxicity,3,7,13 TB might be a better alternative for staining ILM and ERM. Although the preliminary results with the use of TB look promising, its long term safety in vitreoretinal surgery requires further in-depth study.
REFERENCES
- 1.Da Mata AP, Burk SE, Riemann CD, et al. Indocyanine green-assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repair. Ophthalmology 2001;108:1187–92. [DOI] [PubMed] [Google Scholar]
- 2.Gandorfer A, Messmer EM, Ulbig MW, et al. Indocyanine green selectively stains the internal limiting membrane. Am J Ophthalmol 2001;131:387–8. [DOI] [PubMed] [Google Scholar]
- 3.Gandorfer A, Haritoglou C, Gass CA, et al. Indocyanine green-assisted peeling of the internal limiting membrane may cause retinal damage. Am J Ophthalmol 2001;132:431–3. [DOI] [PubMed] [Google Scholar]
- 4.Kwok AK, Li WW, Pang CP, et al. Indocyanine green staining and removal of internal limiting membrane in macular hole surgery: histology and outcome. Am J Ophthalmol 2001;132:178–83. [DOI] [PubMed] [Google Scholar]
- 5.Kadonosono K, Itoh N, Uchio E, et al. Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol 2000;118:1116–8. [DOI] [PubMed] [Google Scholar]
- 6.Sippy BD, Engelbrecht NE, Hubbard GB, et al. Indocyanine green effect on cultured human retinal pigment epithelial cells: implication for macular hole surgery. Am J Ophthalmol 2001;132:433–5. [DOI] [PubMed] [Google Scholar]
- 7.Engelbrecht NE, Freeman J, Sternberg P Jr, et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol 2002;133:89–94. [DOI] [PubMed] [Google Scholar]
- 8.Feron EJ, Veckeneer M, Parys-Van Ginderdeuren R, et al. Typan blue staining of epiretinal membranes in proliferative vitreoretinopathy. Arch Ophthalmol 2002;120:141–4. [DOI] [PubMed] [Google Scholar]
- 9.Melles GR, de Waard PW, Pameyer JH, et al. Typan blue capsule staining to visualize the capsulorhexis in cataract surgery. J Cataract Refract Surg 1999;25:7–9. [DOI] [PubMed] [Google Scholar]
- 10.Eckardt C, Eckardt U, Groos S, et al. [Removal of the internal limiting membrane in macular holes. Clinical and morphological findings.] Ophthalmologe 1997;94:545–51. [DOI] [PubMed] [Google Scholar]
- 11.Hiscott P, Gray R, Grierson I, et al. Cytokeratin-containing cells in proliferative diabetic retinopathy membranes. Br J Ophthalmol 1994;78:219–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiscott P, Wong D, Grierson I. Challenges in ophthalmic pathology: the vitreoretinal membrane biopsy. Eye 2000;14:549–59. [DOI] [PubMed] [Google Scholar]
- 13.Weinberger AW, Kirchhof B, Mazinani BE, et al. Persistent indocyanine green (ICG) fluorescence 6 weeks after intraocular ICG administration for macular hole surgery. Graefes Arch Clin Exp Ophthalmol 2001;239:388–90. [DOI] [PubMed] [Google Scholar]
- 14.Spence DJ, Peyman GA. A new technique for the vital staining of the corneal endothelium. Invest Ophthalmol 1976;15:1000–2. [PubMed] [Google Scholar]
- 15.Hiscott, P. Vitreous biopsy pathology : new kid on the block. Curr Diag Pathol 2001;7:45–55. [Google Scholar]
- 16.Kanawati C, Wong D, Hiscott P, et al. ‘En bloc’ dissection of epimacular membranes using aspiration delamination. Eye 1996;10:47–52. [DOI] [PubMed] [Google Scholar]
- 17.Sethi CS, Lewis GP, Leitner WP, et al. Neuronal plasticity in complicated clinical and experimental retinal detachment (RD). Invest Ophthalmol Vis Sci 2001;42:S445 (Abstract no 2401;42:2001).
- 18.Veckeneer M, van Overdam K, Monzer J, et al. Ocular toxicity study of typan blue injected into the vitreous cavity of rabbit eyes. Graefes Arch Clin Exp Ophthalmol 2001;239:698–704. [DOI] [PubMed] [Google Scholar]
- 19.Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol 1995;119:752–9. [DOI] [PubMed] [Google Scholar]