Abstract
Background/aim: Povidone-iodine (PI, Betadine) is routinely used as a preoperative topical antiseptic in cataract surgery as it has been shown to reduce the incidence of postoperative endophthalmitis. However, the concentration used clinically is variable. In vitro studies have shown that PI is paradoxically more effective at lower concentration. This study was undertaken to determine if this effect was reproducible in vivo.
Methods: A prospective randomised double blind study was carried out in the ophthalmic theatre in a district general hospital. 105 patients attending for routine cataract surgery were randomly allocated to have their conjunctival fornices irrigated preoperatively with either PI 1% (group A) or PI 5% (group B). Conjunctival swabs were taken, in identical fashion, both before and 1 minute after irrigation. The number and species of bacterial colonies cultured from each swab was counted. The difference in the median number of bacterial colonies from pre-irrigation to post-irrigation cultures was then compared between the groups.
Results: Bacterial cultures were gained from 100 patients (33 male, 67 female, mean age 74 years, range 30–95 years). Group B (5% PI) showed a decrease in median colony forming units (CFU) pre-irrigation from 100 to 40 CFU post-irrigation (a drop of 60%). This was greater than in group A (1% PI) where the reduction was 120 CFU pre-irrigation to 100 CFU post-irrigation (a drop of 16.7%) (Mann-Whitney test, p<0.05). At higher initial bacterial loads (CFU pre-irrigation >1000), the difference in median between the two groups became larger as the number of pre-irrigation bacteria increased. In group B pre-irrigation CFU reduced from 3340 to 110 post-irrigation (a drop of 96.7%) compared with group A: 5000 CFU pre-irrigation to 3000 post-irrigation (a drop of 40%) (Mann-Whitney test, p=0.0014).
Conclusion: Despite in vitro evidence of higher bactericidal efficacy of PI at more dilute concentrations, 5% PI is more effective than 1% PI in decreasing the human conjunctival bacterial flora in vivo, particularly in the presence of heavier initial bacterial load.
Keywords: povidone-iodine, endophthalmitis, phacoemulsification, antiseptic
A lthough the incidence of endophthalmitis following cataract surgery is rare at about 0.1%,1,2 it remains a serious postoperative complication with a potentially poor visual prognosis. Various methods of prophylaxis have been used in an effort to minimise the risk of postoperative endophthalmitis, but the designs of studies with sufficient power to measure their efficacy are hampered by the large sample sizes required to produce a statistically significant result. In a recent comprehensive literature review of various prophylactic techniques, Ciulla et al found preoperative irrigation with povidone-iodine (polyvinylpyrrolidone-iodine; PI) to be the most strongly recommended technique based on the current clinical evidence (the strength of povidone-iodine was not specifically mentioned).1
Povidone-iodine has been shown to be effective against a wide range of bacteria, as well as fungi, protozoa, and viruses.3–5 Although some bacteria have demonstrated a “pseudo-resistance” to povidone-iodine, this is presumed to be due to their ability to coat themselves in a protective extracellular matrix.4,6 This inhibition is inversely proportional to the povidone-iodine concentration.7 It is not inhibited by normal saline or water solutions.8
The ideal concentration of povidone-iodine for maximal efficacy is not clarified. Povidone-iodine stock solution is 10%, comprising 90% water, 8.5% povidone-iodine, 1% available iodine, and iodide.3 Previous studies have shown that 5% povidone-iodine effectively decreases the bacterial flora of the ocular surface and adnexae,9–12 and thus theoretically decreases the risk of endophthalmitis, while other large studies have demonstrated 5% povidone-iodine to directly decrease the incidence of endophthalmitis.1,13
More dilute concentrations have been studied in vivo in dogs’ eyes where 0.2% povidone-iodine was shown to be equally as bactericidal as 1% and 5% povidone-iodine.14 In human eyes, in a small study, 0.02% povidone-iodine irrigation has been found to be equally bactericidal compared to 5% povidone-iodine drops.9
There has been no study to compare more dilute concentrations of povidone-iodine with 5% povidone-iodine in the human eye while controlling other variables such as method or length of irrigation. We therefore conducted a prospective randomised double blind comparative study of the effect of 5% povidone-iodine against 1% povidone-iodine on the bacterial flora of the human conjunctiva, using an identical and clinically relevant method of application, to see if the increased bactericidal effect of lower concentrations seen in vitro was reproducible in vivo.
METHODS
Ethical approval for the study was obtained from the Forth Valley Health Board ethics of research committee. The supply of povidone-iodine in randomised aliquots of either 1% or 5% dilution was sourced from a nearby pharmaceutical laboratory. Aliquots were supplied in identical smoked glass bottles, numbered from 1 to 105.
Patients attending for routine cataract surgery at Stirling Royal Infirmary were invited to take part in the study, via a written information sheet accompanying their letter of appointment to attend for pre-assessment. Informed consent was then obtained from those agreeing (105 in total) at the pre-assessment visit 1 week before their operation. Exclusion criteria were current eye infection, use of topical or systemic antimicrobial agents, allergy to iodine, previous intraocular surgery, and pregnancy.
Our standard preoperative preparation was carried out on each patient: three applications of single dose units of proxymetacaine hydrochloride 0.5%, cyclopentolate 1%, phenylephrine 2.5%, and diclofenac sodium 0.1% were applied to the operative eye 1 hour before surgery.
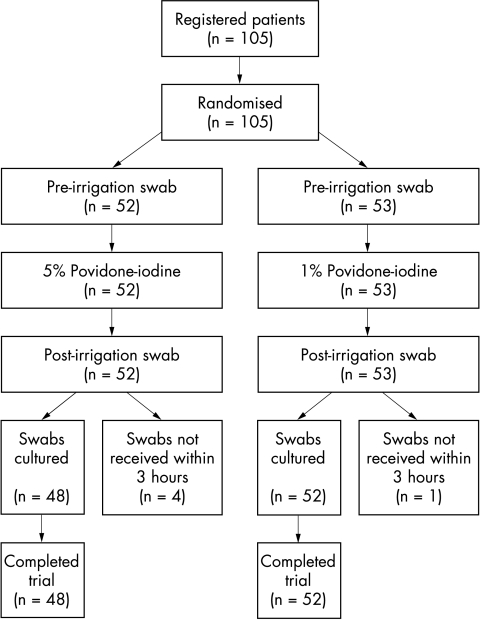
For each participant, a swab from the inferior conjunctival fornix, of the eye to be operated on, was taken with a sterile cotton tipped applicator in the anaesthetic bay before local anaesthesia and surgery. In order to reduce operator sampling bias, a standardised swabbing technique was used for all study patients. The swab was then inoculated in a bijou bottle containing 2 ml of tryptone soya broth with 0.5% sodium thiosulphate broth. The ocular surface of the same eye was then irrigated with one of the randomised aliquots of povidone-iodine by dripping 2 ml of the solution from a syringe directly on to the eye over 1 minute. After a further minute a second swab was taken in identical fashion to the first. Both swabs were labelled with the number of the randomised povidone-iodine aliquot used, as well as “A” or “B” for the pre-irrigation and post-irrigation swabs respectively. The patient’s details were kept separately with the same number. Inoculated swabs were transferred directly to the microbiologist for culture within 3 hours of being taken (see Fig 1). By this method and to reduce bias, the swabber/irrigator and the microbiologist were blinded to the povidone-iodine concentration used.
Figure 1.
Flow chart describing progress of patients through trial.
On completion of the sampling for the study, routine operative protocol was followed: all patients subsequently received local anaesthesia by sub-Tenon’s injection (bupivacaine 0.75% and lignocaine 2%) either inferonasally or inferotemporally. Honan’s balloon was not used. Patients were then taken into the operating room where they received further preoperative cleansing of the ocular surface and periorbital skin with 5% povidone-iodine immediately before surgery, as in the guidelines for cataract surgery issued by the Royal College of Ophthalmologists15 (normal practice for the department is to irrigate the eye with 5% povidone-iodine in the anaesthetic room before and after anaesthesia, with formal re-preparation after transfer to the operating theatre). Patients then proceeded to phacoemulsification and posterior chamber lens implantation.
On reaching the microbiology laboratory, samples were vortexed for 30 seconds. Subsequently, 100 μl aliquots were spread onto: (1) a chocolate blood agar plate which was incubated for 48 hours in 10% carbon dioxide; (2) an anaerobic basal agar containing 5% horse blood which was incubated for 48 hours in an anaerobic cabinet (Na 80%, H2 10%, CO2 10%); after which colony forming units (CFU) were counted in both plates. A further 100 μl was incubated into fastidious anaerobic broth that was incubated for 7 days, and then subcultured anaerobically and in 10% carbon dioxide.
Colonies were counted by hand, using an illuminated colony counter when large numbers of colonies were present. The number of colonies on each plate was converted to number of bacteria per 2 ml of tryptone soya broth (equal to number of bacteria per eye) using the equation:
Total CFU per eye = (CFU on plate per amount of solution plated) × (volume of original solution)
Bacterial species were identified using conventional biochemical and biophysical reactions.
The sample size of 100 had 80% power to detect as significant at the 5% level a true mean difference in normally distributed outcomes of 0.65 standard deviations. For counts of CFU, which were approximately normally distributed after logarithmic transformation, this corresponded to a fourfold change in levels. To enable logarithmic transformation a count of 10 was arbitrarily assigned when no CFU were detected (being less than half the minimum detectable CFU count, and where the number of CFU was too large to count (that is, CFU >8000) a count of 16 000 was assigned (that is, double the maximum countable number). Numbers of CFU counted ranged from 10 to 16 000 after logarithmic transformation in each treatment group, both before and after irrigation. Raw data were used for qualitative analysis, but logarithmic transformation was employed for quantitative statistical data analysis to correct the extreme skewness in these numbers.
The two treatment groups were compared using Mann-Whitney tests for numbers of CFU; and χ2 tests with Yates’s correction for presence or absence of specific bacteria. Multiple linear regressions were used to compare the two groups between pre-irrigation and post-irrigation CFU, using the logarithms of the counts.
RESULTS
In all, 105 patients were recruited, but the swabs from five patients were not received by the laboratory within 3 hours of sampling and so were not cultured and therefore excluded (see Fig 1). The code for the correlation of patient with the dilution of povidone-iodine used for each patient was not broken until all microbiological data were complete.
The results of the pre-irrigation and post-irrigation cultures on 100 patients were available for analysis; 67 patients were female and 33 were male. The mean age was 74 years (range 30–95; SD 10.4 years). Forty eight patients received 5% povidone-iodine and 52 patients received 1% povidone-iodine. The two groups showed no statistical difference with respect to age (p=0.7, unpaired t test) or sex (p>0.999, Yates’s corrected χ2).
No patient in the study developed postoperative endophthalmitis or surgical complication as a result of the study, nor suffered any adverse reaction to the irrigation fluid or swabbing procedure.
Qualitative data
The number of CFU decreased following irrigation with povidone-iodine in 84 of the 100 cultures. Of the 16 cultures that showed an increase in CFU, 10 were in the 1% group and six in the 5% group (no statistical difference between the groups). The median CFU in the 1% PI group changed from 120 before irrigation to 100 after irrigation (a drop of 16.7%) and from 100 before irrigation to 40 after irrigation in the 5% PI group (a drop of 60%). The difference in post-irrigation CFU between the two groups was significant (Mann-Whitney U; p<0.05).
Quantitative data
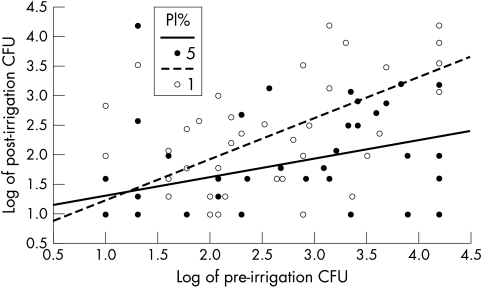
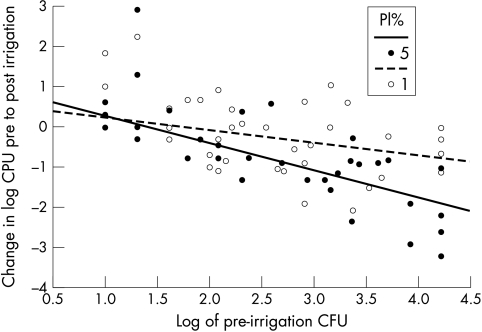
As the CFU varied over four orders of magnitude, further analysis was done after logarithmic transformation, and Figure 2 shows a plot of (log) post-irrigation CFU against pre-irrigation CFU, with separate regression lines fitted to each group. An interaction test in a multiple linear regression showed that the slope of the line for the 5% PI group was significantly less than that for the 1% PI group (t = 2.79, 96 degrees of freedom, p=0.006). Multiple linear regression analysis of the change in log CFU, showed that the gradient of the line for 5% PI was significantly steeper in this case (Fig 3). This implies that the 5% PI dosage was especially effective relative to the 1% dose in the context of high initial levels of CFU. Indeed, among those with pre-irrigation CFU >1000, the difference was even more significant: 1% PI subgroup median CFU changed from 5000 pre-irrigation to 3000 post-irrigation (40% reduction); and 5% PI subgroup changed from 3340 pre-irrigation to 110 post-irrigation (96.7% reduction) (p=0.0014). Conversely, the difference in CFU in the subgroup with pre-irrigation CFU <1000 showed no statistically significant difference (p=0.12)
Figure 2.
Plot of post-irrigation against pre-irrigation CFU on a logarithmic scale to base 10.
Figure 3.
Plot of change in logarithm to base 10 of CFU between pre-irrigation and post-irrigation assessments against logarithm of pre-irrigation CFU.
Bacterial species
Table 1 summarises the results for prevalence of bacteria species in each treatment group before and after irrigation. The type of bacteria isolated were consistent with the bacterial flora found in previous studies.9–12 None of these either before or after irrigation showed a significant difference between the treatment groups. Twenty six of the 100 cultures were “sterile” (yielded no cultured organism) before irrigation, and 22 of these were also “sterile” following irrigation. The total number of “sterile” cultures post-irrigation was 34. Where post-irrigation bacteria were present, the same species were also present in the pre-irrigation cultures in 95 of 100 cultures. Coagulase negative staphylococci (CNS) were present pre-irrigation and post-irrigation in 29 patients (88%) treated with 1% PI and 18 (69%) of those treated with 5% PI; this difference between the two groups approached significance (Yates, p=0.07), while the counts for post-irrigation CNS without pre-irrigation CNS were low in both groups at 2 (10%) and 1 (4%) respectively.
Table 1.
Number (%) of patients with specific bacteria in each treatment group before and after irrigation
| Pre-irrigation | Post-irrigation | |||
| 1% PI | 5% PI | 1% PI | 5% PI | |
| Coagulase negative staphylococci | 33 (63) | 26 (54) | 31 (60) | 19 (40) |
| Micrococcus | 6 (11) | 5 (10) | 4 (8) | 3 (6) |
| Moraxella | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| Proteus | 3 (6) | 2 (4) | 3 (6) | 1 (2) |
| Staph aureus | 5 (10) | 9 (19) | 5 (10) | 5 (10) |
| α Haemolytic streptococci | 5 (10) | 5 (10) | 2 (4) | 3 (6) |
| Corynebacterium | 2 (4) | 1 (2) | 0 (0) | 1 (2) |
| Peptococcus | 1 (2) | 2 (4) | 1 (2) | 0 (0) |
| Klebsiella | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| E coli | 1 (2) | 0 (0) | 1 (2) | 0 (0) |
DISCUSSION
Povidone-iodine has been shown to be bactericidal against a wide range of bacteria, and is also effective against fungi, protozoa, and viruses.3–5 Povidone is hydrophilic and acts as a carrier of the iodine moiety to cell membranes. Once the povidone-iodine complex reaches the cell wall, the free iodine released is rapidly cytotoxic, killing the prokaryotic cell within 10 seconds.4 Further free iodine is released from the povidone-iodine complex as free iodine is used up, until the available iodine is exhausted. The free iodine concentration has been shown to increase with more dilute concentrations of povidone-iodine, with a maximal free iodine concentration of 24 parts per million at 0.7%.3 This paradoxical effect follows a “bell curve”: concentrations less than 0.05% lose their povidone-iodine complex characteristics and behave like aqueous iodine. Correspondingly, the in vitro bactericidal efficacy of povidone-iodine has been shown to increase at more dilute concentrations of 0.1 to1%, with relatively faster killing rates.16
Previous studies have shown that 5% povidone-iodine effectively decreases the bacterial flora of the ocular surface and adnexae,9–12 and thus theoretically decreases the risk of endophthalmitis. Other large studies have demonstrated 5% povidone-iodine to directly decrease the incidence of endophthalmitis, although, as noted by the authors, the design of these studies is not ideal: Schmitz et al acknowledge the limitations of their retrospective survey design17; Speaker and Menikoff conducted a prospective parallel trial, however it was not randomised and antibiotic prophylaxis was an uncontrolled variable.13
Our results show a significant difference in bactericidal activity in vivo between 5% and 1% povidone-iodine, with 5% povidone-iodine demonstrating more activity overall. Interestingly, there is no statistical difference between the two strengths with low initial bacterial loads—the difference becomes more marked only as the initial load of bacteria increases. This is in contrast with results seen in vitro.16 In vivo, known inhibitors of povidone-iodine (blood, pus, fat, glove powder7 as well as protein containing solutions8) may be present and may have a role of altering bactericidal efficacy, or the dose or volume of the povidone-iodine may vary depending on the contact time and retention within the conjunctival fornix.
Nevertheless, Roberts et al demonstrated, in dogs’ eyes in vivo, that 0.2% povidone-iodine (continuous ocular irrigation and periocular scrub for 2 minutes followed by soak for 2 minutes) was equally as bactericidal as 1% and 5% povidone-iodine.14 Grimes et al, in a small study of human eyes of 22 patients, again found 0.02% povidone-iodine irrigation (duration not specified) to be equally bactericidal as 5% povidone-iodine drops.9 The discrepancies between our results and previous studies may be explained by the povidone-iodine concentration, or the mode or duration of application. Povidone-iodine 1%, although initially more bactericidal, has a lower reservoir of available iodine which is exhausted when the bacterial load is increased. The study in dogs’ eyes14 irrigated the ocular surface with povidone-iodine for a total of 4 minutes (compared to 1 minute in our study), which would allow the available iodine reservoir to be continually replenished and so avoid this problem. We used 1 minute as our time of irrigation as this was closer to the actual time we currently spend irrigating the ocular surface in the anaesthetic room (although the total time the povidone-iodine is in contact with the ocular surface before the operation commences is approximately 4–5 minutes). Irrigating the ocular surface for a longer period may therefore show an improvement in the performance of 1% povidone-iodine (with results similar to those seen in the dogs’ eye study). Confirmation of the minimum time of irrigation for each concentration would need to be studied with further prospective randomised studies and was outside the scope and resources of our study. There may be an optimum concentration/time balance which provides acceptable reduction in CFU count, in a reasonable and practical application time without ocular toxicity.
Our results do raise the question of whether an even higher (for example, 10%) concentration would prove even more effective as a bactericidal agent and in a shorter time, but at the risk of toxicity. In many units, 5% povidone-iodine is diluted from hospital stock solution (10%) povidone-iodine (Betadine). The choice of 5% povidone-iodine, as opposed to the 10% stock solution, was based on concerns over the toxicity of the undiluted form12 and the evidence base to support the use of 5% povidone-iodine. The comparative bactericidal effect of the stock solution (10%) povidone-iodine was not studied in this trial. This product has a typical free iodine concentration of one part per million (0.0001 %), being in a state of dynamic equilibrium with the povidone-iodine complex.
The documented toxicity of topical povidone-iodine is largely limited to conjunctival irritation (incidence of 0.4%)3 (and from one of the author’s personal experience certainly most unpleasant in an unanaesthetised eye!). Keratoconjunctivitis sicca has also been reported.18 Contact dermatitis is less common (0.04%); however, the risk of a reaction is increased tenfold in the presence of allergy to shellfish or iodine.3 Although it is not common, the incidence of a conjunctival reaction seems to be directly related to the concentration of povidone-iodine used.12,18 This may be explained by the pH of povidone-iodine solution, which becomes less acidic with dilution14,16 and thus more closely approximates the pH of the conjunctiva.
Wille evaluated corneal swelling and endothelial cell loss with specular microscopy following cataract surgery; he did not show any increased corneal damage when povidone-iodine was used.19 Unfortunately the strength of povidone-iodine used was not mentioned in the study. MacCrae et al studied rabbit corneas after application of 10% povidone-iodine and showed moderate transient corneal oedema at 5 minutes, which had resolved by 3 hours,20 while Tsunoda found the cytotoxicity of povidone-iodine in vivo in rats was less than in vitro.21
The cytotoxicity of povidone-iodine on fibroblasts and polymorphonuclear lymphocytes is also directly related to the concentration,3,14 with concentrations as low as 0.5% retarding wound healing in rabbit models by 24 hours.22 Intravitreal injection of povidone-iodine in rabbit eyes causes retinal oedema and necrosis, again in a dose dependent fashion23 and therefore intraocular contamination must be viewed with concern. Establishing the correct therapeutic ratio of concentration dose and time is important, and reducing concentration of the irrigating fluid would be seen as an advantage, but not at the expense of inadequate bacterial kill.
We chose to take our samples before the injection of any local anaesthesia as povidone-iodine is known to be inhibited by blood,7 and in our experience a small amount of subconjunctival haemorrhage is not uncommon following sub-Tenon’s injection. This inhibition is worth considering in current preoperative antisepsis methods (regardless of strength used) as our study shows residual conjunctival bacteria present in 66% of post-irrigation cultures. It would therefore seem prudent to irrigate the ocular surface before local anaesthesia to avoid inhibition of povidone-iodine and thus minimise the presence of conjunctival bacteria, and to extend the effective time before surgical entry into the eye.
A total of 16% of cultures showed an increase in the number of bacteria following irrigation, with 4% showing a new species. These cases occurred in both groups, which would indicate this result may an artefact. Possible sources would be sampling errors of small numbers of bacteria missed by the first swab, or mechanical release of bacteria from the lid margins by the mechanical action of taking the swab. This effect has been noted in previous studies where irrigation with normal saline has caused an increase in the number of bacterial species cultured.24 We have included all culture results in our analysis none the less.
None of our patients developed postoperative endophthalmitis, but the study is of too low a power to draw conclusions from this. A truly sterile conjunctival fornix is probably not achievable, but reduced external load probably reduces anterior chamber contamination and allows natural defence mechanisms (for example, defensins) not to become overloaded.
SUMMARY
In conclusion therefore, this study supports the use of 5% povidone-iodine in everyday clinical use. Up to 96.7% bacterial kill is achieved with only 1 minute of irrigation. Despite in vitro evidence to the contrary, with a short irrigation time 5% povidone-iodine is more effective than 1%, particularly in the presence of large numbers of bacteria. Exact times and concentrations of povidone-iodine to establish optimum therapeutic ratios require further studies.
Acknowledgments
This study was supported by a grant from the Research and Development Fund of the Forth Valley Acute Hospitals NHS Trust. The authors wish to thank Professor P Aspinall for statistical advice while devising the study; Dr K Kumar and Dr C Scarlett for their contributions in data collection; Drs JD Huggan, AT Crawford, JA Scott, and T Saboor for permission to include patients under their care; Maisie Martindale, clinical pharmacist at Stirling Royal Infirmary, and Tayside Pharmaceuticals at Ninewells Hospital, Dundee for the production of the test solutions, and the staff of the ophthalmic theatre at Stirling Royal Infirmary for their patience.
Footnotes
Funding: This study was supported by a grant from the Forth Valley Acute Hospitals Research and Development fund.
Competing interest: None.
This study was presented at the annual meeting of the Scottish Ophthalmological Club on 8 March 2002.
The authors declare no competing or proprietary interest in Betadine or other products.
REFERENCES
- 1.Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery. An evidence-based update. Ophthalmology 2002;109:13–24. [DOI] [PubMed] [Google Scholar]
- 2.Scottish Intercollegiate Guidelines Network. Day case cataract surgery SIGN guidelines. Edinburgh: Scottish Intercollegiate Guidelines Network, 2001:51.
- 3.Zamora JL. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am J Surg 1986;151:400–6. [DOI] [PubMed] [Google Scholar]
- 4.Lacey RW, Catto A. Action of povidone-iodine against methicillin-sensitive and -resistant cultures of Staphylococcus aureus. Postgrad Med J 1993;69:S78–83. [PMC free article] [PubMed] [Google Scholar]
- 5.Prince HN, Nonemaker WS, Norgard RC, et al. Drug resistance studies with topical antiseptics. J Pharm Sci 1978;67:1629–31. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RL, Vess RW, Carr JH, et al. Investigations of intrinsic Pseudomonas cepacia contamination in commercially manufactured povidone-iodine. Infect Control Hosp Epidemiol 1991;12:297–302. [DOI] [PubMed] [Google Scholar]
- 7.Zamora JL, Price MF, Chuang P, et al. Inhibition of povidone-iodine’s bactericidal activity by common organic substances: an experimental study. Surgery 1985;98:25–9. [PubMed] [Google Scholar]
- 8.Davis GHG, Finlayson N, Kemp R. Dilution of povidone-iodine (letter). Med J Aust 1985;143:321. [DOI] [PubMed] [Google Scholar]
- 9.Grimes SR, Hollsten D, Nauschuetz WF, et al. Effect of povidone-iodine on the pre-operative chemical preparation of the eye. Military Med 1992;157:111–13. [PubMed] [Google Scholar]
- 10.Dereklis DL, Bufidis TA, Tsiakiri EP, et al. Preoperative ocular disinfection by the use of povidone-iodine 5%. Acta Ophthalmol (Copenh) 1994;72:627–30. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell DR, Kastl PR, Cook J, et al. Povidone-iodine: its efficacy as a preoperative conjunctival and periocular preparation. Ann Ophthalmol 1984;16:577–80. [PubMed] [Google Scholar]
- 12.Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery III. Effect of povidone-iodine on the conjunctiva. Arch Ophthalmol 1984;102:728–9. [DOI] [PubMed] [Google Scholar]
- 13.Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology 1991;98:1769–75. [DOI] [PubMed] [Google Scholar]
- 14.Roberts SM, Severin GA, Lavach JD. Antibacterial activity of dilute povidone-iodine solutions used for ocular surface disinfection in dogs. Am J Vet Res 1986;47:1207–10. [PubMed] [Google Scholar]
- 15.The Royal College of Ophthalmologists. Cataract surgery guidelines. London: RCO, 2001.
- 16.Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol 1982;15:635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz S, Dick HB, Krummenauer F, et al. Endophthalmitis in cataract surgery: results of a German survey. Ophthalmology 1999;106:1869–77. [DOI] [PubMed] [Google Scholar]
- 18.Gills JP. Effective concentration of Betadine (letter). J Cataract Refract Surg 1999;25:604. [PubMed] [Google Scholar]
- 19.Wille H. Assessment of possible toxic effects of polyvinylpyrrolidone-iodine upon the human eye in conjunction with cataract extraction. Acta Ophthalmol (Copenh) 1982;60:955–60. [DOI] [PubMed] [Google Scholar]
- 20.MacRae SM, Brown B, Edelhauser HF. The corneal toxicity of presurgical skin antiseptics. Am J Ophthalmol 1984;97:221–32. [DOI] [PubMed] [Google Scholar]
- 21.Tsunoda A, Shibusawa M, Tsunoda Y, et al. Implantation on the suture material and efficacy of povidone-iodine solution. Eur Surg Res 1997;29:473–80. [DOI] [PubMed] [Google Scholar]
- 22.York KK, Miller S, Gaster RN, et al. Polyvinylpyrrolidone iodine: corneal toxicology and epithelial healing in a rabbit model. J Ocul Pharmacol 1988;4:351–8. [DOI] [PubMed] [Google Scholar]
- 23.Whitacre MM, Crockett RS. Tolerance of intravitreal povidone-iodine in rabbit eyes. Curr Eye Res 1990;9:725–32. [DOI] [PubMed] [Google Scholar]
- 24.Isenberg S, Apt L, Yoshimori R. Chemical preparation of the eye in ophthalmic surgery I. Effect of conjunctival irrigation. Arch Ophthalmol 1983;101:761–3. [DOI] [PubMed] [Google Scholar]