Abstract
Aim: To investigate the relation between intercellular adhesion molecule (ICAM)-1 expression and cellular dynamics occurring concomitantly with epithelial cell movement.
Methods: Outgrowing epithelial sheets of human corneal epithelial (HCE) cells from cultured limbal explants were examined by immunoperoxidase staining with anti-ICAM-1 monoclonal antibody. An adhesion assay was performed using the epithelial sheets of HCE cells and an Epstein-Barr virus (EVB) infected B cell lymphoma cell line (EVB+BJAB) expressing CD11a/CD18, a counter-receptor of ICAM-1. Also, the effect of calphostin C, a specific protein kinase C (PKC) inhibitor, on ICAM-1 expression on the outgrowing epithelial sheets of HCE cells was examined.
Results: Strong positive staining for ICAM-1 was found predominantly on HCE cells in the marginal segment of the epithelial sheet, particularly on the cells at the leading edge. EBV+BJAB cells adhering to the HCE cells corresponded well to the area of ICAM-1 staining. Treatment of outgrowing epithelial sheets with calphostin C markedly decreased the ICAM-1 expression on the HCE cells.
Conclusion: ICAM-1 is actively expressed on HCE cells in the marginal segment of the outgrowing epithelial sheets where there is active movement mediated through a PKC dependent mechanism, suggesting the role of ICAM-1 in epithelial cell motility such as the spreading and migration of cells.
Keywords: cell movement, human corneal epithelial cell, intercellular adhesion molecule-1, protein kinase C
Intercellular adhesion molecule-1 (ICAM-1, CD54) is a transmembrane glycoprotein with a molecular weight of 80 000 to 110 000, functioning as an adhesion molecule in a variety of biological situations.1 One previous study revealed the association of ICAM-1 molecule with the actin containing cytoskeletal proteins in COS-7, a monkey kidney epithelial cell line, and in Epstein-Barr virus transformed B cells.2 Linkage between transmembrane proteins and cytoskeletal proteins is critical for various cellular events such as cell to extracellular matrix (ECM) interaction and cell motility. In addition, ICAM-1 has been shown to serve as a receptor for hyaluronic acid (HA),3 a polysaccharide, to provide a cell free space into which cells can migrate.4 Interaction between HA and its receptor has been suggested to promote locomotion.5 Although neither the role of cytoskeletal interaction in ICAM-1 function nor the biological significance of ICAM-1 as a receptor for HA has been clarified yet, those previous studies suggested that a linkage of ICAM-1 to the cytoskeletal proteins facilitates not only a strong cell to cell adhesion but also cell to ECM or cell to substratum interactions required for cell motility. For the past decade, extensive studies have demonstrated the functions of ICAM-1 as an adhesion receptor that binds to its counter-receptors, lymphocyte function associated antigen-1 (LFA-1, CD11a/CD18), and Mac-1 (CD11b/CD18),6,7 to promote a variety of cell to cell interactions. However, the functions of ICAM-1 in cell motility such as cell spreading and migration have not been thoroughly investigated.
Cultured HCE cells show various cellular activities—for example, the synthesis of cytoskeletal proteins,8 and active cell spreading and migration as well as those in their wound healing process. Although ICAM-1 is not expressed on normal corneal epithelium, our previous study demonstrated the expression of ICAM-1 on cultured human corneal epithelial (HCE) cells.9 Therefore, there is a possibility that ICAM-1 is expressed preferentially on HCE cells in their active movement. In primary cultured HCE cells obtained from limbal explant, HCE cells overgrow and migrate on culture dishes and form a coherent sheet with an incubation period.10 Therefore, in the present study, we investigated the relation between ICAM-1 expression and corneal epithelial cell movement, using this primary HCE cell culture system to investigate the role of ICAM-1 in cellular dynamics and the mechanism of its function.
MATERIALS
HCE cell culture
Human corneas for corneal transplantation were provided by domestic local eye banks in Japan. The residual of corneal tissue after corneal transplantation, consisting of peripheral cornea with limbus, termed a corneal rim, was used in the present study. This study was approved by the ethics committee for human research in Nihon University.
The corneal rim was dissected along the stromal lamella and the upper portion with epithelium was cut into 16 blocks. Each block was used as a limbal explant in this study. Each explant was placed epithelial side up on a chamber slide (Laboratory-Tek 2 well Permanox Slide, 177429; Nunc, Naperville, IL, USA) and left covered for approximately 15 minutes until the explant attached firmly to the slide. Then, 2 ml of modified supplemental hormonal epithelial medium (SHEM) was added to each chamber, followed by incubation at 37°C under 5% carbon dioxide. Modified SHEM consisted of Ham’s F12 and Dulbecco’s modified Eagle medium (DMEM; 1:1; Gibco BRL, Grand Island, NY, USA) containing mouse epidermal growth factor (10 ng/ml), bovine insulin (5 μg/ml; Gibco BRL), cholera toxin (0.1 μg/ml), dimethylsulfoxide (0.5%; Sigma, St Louis, MO, USA), gentamicin (40 μg/ml; Schering-Plough, Osaka, Japan), penicillin G (100 U/ml; Banyu Pharmaceutical, Tokyo, Japan), and 10% fetal bovine serum (FBS, Gibco BRL). The medium was changed every 3 days. Outgrowing cells from explants were confirmed as epithelial cells by epithelial keratin expression, and contamination by Langerhans cells or corneal stromal cells was excluded by the previously described method.11
Treatment of HCE cells of epithelial outgrowths with proinflammatory cytokines and a protein kinase C inhibitor
Some epithelial outgrowths on wells of the chamber slides were treated with either recombinant human IFN-γ (1000 U/ml) or TNF-α (2000 U/ml; Genzyme, Cambridge, MA, USA) for 3 days and stained by immunoperoxidase technique (described in a later section). A portion of the epithelial outgrowths was treated with calphostin C (Sigma), a specific PKC inhibitor, at concentrations ranging from 0 to 4 μmol for 1 hour, then washed with medium thoroughly, followed by incubation with modified SHEM for 2 days just before immunostaining.
Immunocytochemical study
Explants were embedded in optimal cutting temperature (OCT) compound (Tissue-Tek; Miles Scientific, Naperville, IL, USA) at 20°C. Frozen OCT embedded sections were cut at 7 μm thickness and placed on poly-l-lysine coated microscope slides (Muto Pure Chemicals, Tokyo, Japan). These OCT sections of explants and epithelial outgrowths on the chamber slides, with or without treatment as described above, were stained by the avidin-biotin peroxidase complex (ABC) method as follows. The plates were fixed in chilled acetone for 8 minutes. Next, they were incubated with blocking serum for 30 minutes, followed by incubation with anti-ICAM-1 monoclonal antibody (mAb; B-C14; Serotec, Oxford, UK) or control mouse IgG (Dako, Glostrup, Denmark) for 60 minutes, and with affinity purified biotinylated horse anti-mouse IgG for 30 minutes. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for 30 minutes, followed by incubation with ABC reagent (Vectastain ABC kit; Vector Laboratories, Burlingame, CA, USA) for 60 minutes. The plates were incubated with 3-amino-9-ethylcarbazole (AEC; Sigma) for 15 minutes and counterstained with Gill’s haematoxylin (Vector Laboratories). All plates were examined by light microscopy (Olympus BH-2; Olympus, Tokyo, Japan).
Adhesion assay
EBV+BJAB/B958, an EBV infected B cell lymphoma cell line, was prepared for adhesion assay as apposing cells expressing LFA-1, a counter-receptor of ICAM-1.12,13 EBV+BJAB cells were suspended in DMEM containing 10% heat inactivated (at 56°C, for 30 minutes) FBS (complete medium). Epithelial outgrowths around 7 days of culture were prepared on the chamber slides as described earlier. EBV+BJAB cells (1 × 105) were added to each chamber in a total volume of 1 ml with complete medium, then incubated at 37°C in 5% carbon dioxide for 1 hour. The chamber slides were washed four times with warm medium to remove EBV+BJAB cells which did not bind to HCE cells. The chamber slides were examined with a phase contrast light microscope (Olympus CK2, Olympus).
Blocking of EBV+BJAB cell adhesion to HCE cells with monoclonal antibodies
Epithelial outgrowths were preincubated with 25 μg/ml of anti-ICAM-1 mAb (84H10; AMAC, Westbrook, ME, USA) or control mouse IgG (Dako) at 37°C in 5% carbon dioxide for 1 hour and then washed three times with complete medium before the addition of EBV+BJAB cells. In some experiments, the EBV+BJAB cells were pretreated with 25 μg/ml of anti-LFA-1 (25.3.1; AMAC) at 37°C in 5% carbon dioxide for 30 minutes. This concentration of mAbs was required for the maximum blocking effect as we previously demonstrated.9 After three time washing with complete medium, these treated EBV+BJAB cells were resuspended in complete medium. Adhesion assays were performed using EBV+BJAB cells and the HCE cells of epithelial outgrowths with or without treatment of mAbs as described earlier. The chamber slides were examined with phase contrast light microscope (Olympus CK2).
RESULTS
ICAM-1 expression on HCE cells from limbal explants in culture
Limbal explants were incubated in Lab-Tek chamber slides with modified SHEM. Epithelial outgrowth from the explants could be observed after 2–3 days of incubation. The area of epithelial outgrowth was extended with lengthening incubation period. After about 7 days of incubation, epithelial outgrowth could be divided into three zones, leading zone, peripheral zone, and central zone, according to both the distance from the explant and the shape of the component epithelial cells (Fig 1). The leading zone was located in the marginal segment of epithelial outgrowth and mainly consisted of large and elongated cells. The peripheral zone was located in the inner segment adjacent to the leading zone and consisted of large cells. The central zone was located in the segment proximate to the explant and consisted of packed small cells. Cells of the epithelial outgrowth were stained by the ABC method after about 7 days of incubation. As shown in Figure 2, positive staining for ICAM-1 was found predominantly on the HCE cells in the leading zone. In addition, the most intense staining for ICAM-1 was found on the HCE cells at the leading edge. In contrast, on the HCE cells in the peripheral zone, positive staining was found much less frequently. In the central zone, ICAM-1 positive cells were rarely found. No large variation in staining pattern of ICAM-1 was observed on epithelial outgrowths among the different portions of the same corneal rim, and no interindividual variation was found, although epithelial outgrowths from 10 different donor corneal tissues were examined.
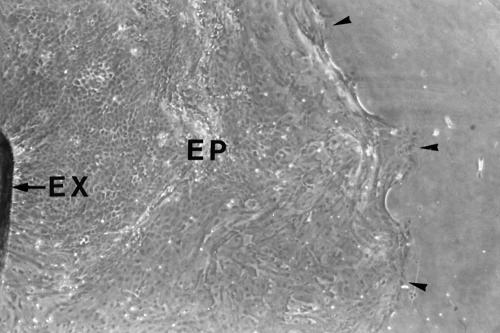
Figure 1.
Human corneal epithelial outgrowth from limbal explant in culture. The limbal explant (EX) was incubated with modified SHEM at 37°C under 5% carbon dioxide for 7 days. Phase contrast micrograph. Original magnification, ×10. Outgrowing epithelial cell sheet (EP). Leading edge of epithelial outgrowth (arrowheads).
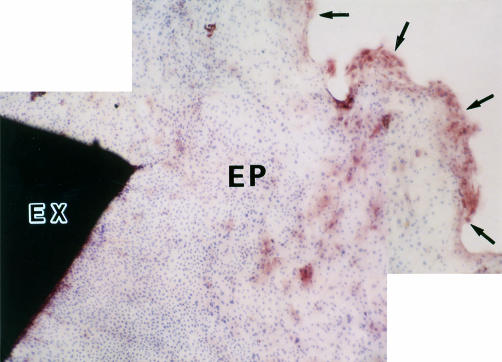
Figure 2.
Immunocytochemical study of ICAM-1 expression on HCE cells. HCE cells of epithelial outgrowth from the limbal explant after 7 days of incubation were stained with anti-ICAM-1 mAb by the ABC method, visualised with AEC, and counterstained with haematoxylin. Positive staining for ICAM-1 (brown stain) was found predominantly on HCE cells in the leading zone. Original magnification, × 34. Limbal explant (EX). Epithelial outgrowth (EP). Leading edge of epithelial outgrowth (arrows).
Frozen OCT sections of corneal rims from 10 donor eyes were stained by the ABC method with anti-ICAM-1 mAb. There was no positive staining for ICAM-1 on corneal epithelial cells as we demonstrated previously (data not shown).9
ICAM-1 expression on HCE cells treated with IFN-γ or TNF-α
There was a possibility that such ICAM-1 expression as demonstrated in this study may be caused by a ICAM-1 inducer which was contained or secreted from the cells in culture medium. To test out the possibility, cells of epithelial outgrowths were treated with recombinant human IFN-γ (1000 U/ml) or TNF-α (2000 U/ml) for 3 days, which treatment was previously shown to induce ICAM-1 expression on cultured HCE cells markedly,9 and stained with anti-ICAM-1 mAb by the ABC method. Basically, the staining patterns on the epithelial outgrowths treated with both cytokines were similar, as follows; the positive staining for ICAM-1 was observed much more diffusely and frequently throughout the epithelial outgrowth than on those cell specimens without any cytokine treatment (Fig 3).
Figure 3.
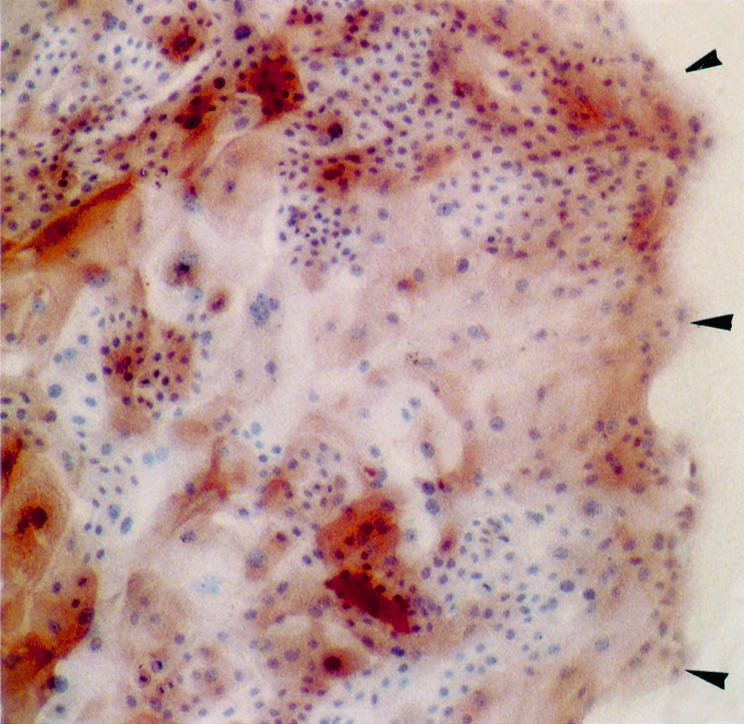
ICAM-1 expression on TNF-α treated HCE cells from the limbal explant. HCE cells from the limbal explant were treated with recombinant human TNF-α (1000 U/ml) for 3 days and stained with anti-ICAM-1 mAb by the ABC method. Original magnification, × 34. Positively and intensely stained cells for ICAM-1 were observed throughout the epithelial sheet. Leading edge of epithelial outgrowth (arrowheads).
EBV+BJAB cell adhesion to HCE cells
We performed adhesion assays using the epithelial outgrowths and EBV+BJAB/B958 cells with high LFA-1 expression, to determine whether ICAM-1 was functionally expressed on HCE cells in the epithelial outgrowths. EBV+BJAB cells adhering to HCE cells were found predominantly in the leading zone; most frequently at the leading edge (Fig 4B). In contrast, adhered EBV+BJAB cells were found much less frequently in the peripheral zone and were rarely found in the central zone (Fig 4A and 4B). In addition, we performed experiments of blocking the adhesion of EBV+BJAB cells to HCE cells of epithelial outgrowth with anti-ICAM-1 and anti-LFA-1 mAbs, to determine whether EBV+BJAB cell adhesion to HCE cells was mediated through ICAM-1 binding to LFA-1. The adhesion of EBV+BJAB cells to HCE cells was efficiently blocked by pretreatment of HCE cells with anti-ICAM-1 mAb or pretreatment of EBV+BJAB cells with anti-LFA-1 mAb, or by combining these treatments (Fig 5).
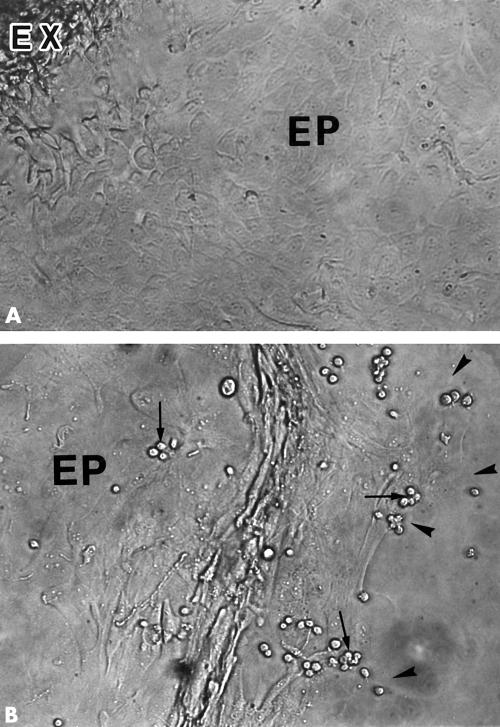
Figure 4.
EBV+BJAB cell adhesion to HCE cells. LFA-1 expressing EBV+BJAB cells were added to HCE cells on a chamber slide and incubated for 1 hour. (A) Central zone. (B) Peripheral and leading zone. EBV+BJAB cells adhering to HCE cells (arrows) were found predominantly in the leading zone of epithelial outgrowth (EP). Limbal explant (EX). Leading edge (arrowheads). Original magnification, ×25.
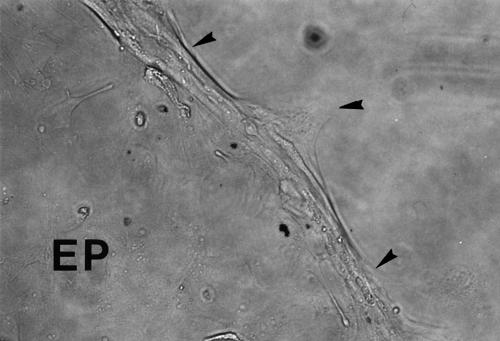
Figure 5.
Blocking of EBV+BJAB cell adhesion to HCE cells. Combining pretreatments of HCE cells and EBV+BJAB cells with anti-ICAM-1 mAb and with anti-LFA-1 mAb, respectively, efficiently blocked the adhesion of EBV+BJAB cells to HCE cells. Phase contrast micrograph. Original magnification, × 25. Leading edge (arrowheads).
Effect of protein kinase C inhibitor on ICAM-1 expression
According to the previous studies, PKC is a crucial enzyme for corneal epithelial cell movement.14,15 In addition, the activation of PKC has been shown to increase ICAM-1 expression on vascular endothelial cells.16 Therefore, to investigate a possible mechanism of the predominant ICAM-1 expression on the HCE cells in the leading zone, we determined the role of PKC in the expression of ICAM-1 by blocking PKC activity using calphostin C, a specific PKC inhibitor.17 The expression of ICAM-1 decreased markedly with 2–4 μmol of calphostin C treatment on the HCE cells of epithelial outgrowths (Fig 6). We confirmed that the result is reproducible by repeating the same experiment with epithelial outgrowths from five different donor corneal tissues.
Figure 6.
Effect of PKC inhibitor on ICAM-1 expression. HCE cells from the limbal explant were treated with 4 μmol of calphostin C, a specific PKC inhibitor, for 1 hour, followed by 2 days of incubation with modified SHEM and immunostaining with anti-ICAM-1 mAb. Immunoreactivity for ICAM-1 (brown stain) decreased markedly on calphostin C treated HCE cells (A) compared to that on non-treated HCE cells (B) in the leading zone. Original magnification, ×85.
DISCUSSION
On the basis of previous studies, we sought to determine the relation between ICAM-1 expression and cellular dynamics occurring concomitantly with epithelial cell movement. In this study we investigated the expression of ICAM-1 on outgrowing epithelial sheets of HCE cells from the limbal explant in culture. Our experimental protocol was based on the previous evidence as follows. Epithelial cells in sheet form are capable of active spreading movements in in vitro cell culture as well as in epithelial wound healing18,19; outgrowth of an epithelial cell sheet from the limbal explant spreads rapidly as an intact coherent sheet in culture,10,20 which provides a good source for the study of cellular events occurring in outgrowing epithelial sheets. We showed in the immunocytochemical study that strong positive staining for ICAM-1 was found predominantly on the HCE cells in the leading zone, which were located in the marginal segment of epithelial outgrowth. The most intense staining for ICAM-1 was found on the HCE cells at the leading edge. In contrast, ICAM-1+cells were found much less frequently in the peripheral zone and rarely found in the central zones, located in the inner segment of the outgrowth and the segment proximate to the explant, respectively. In addition to immunocytochemical study, we performed adhesion assays using epithelial outgrowth of HCE cells and EBV+BJAB cells with high LFA-1 expression on the cell surface, to confirm that ICAM-1 is functionally expressed on the cell surface. EBV+BJAB cells adhering to the HCE cells were found predominantly in the leading zone and most frequently at the leading edge, corresponding well to the findings in our immunocytochemical study. Moreover, the adhesion of EBV+BJAB cells to the HCE cells was blocked efficiently either by pretreatment of HCE cells with anti-ICAM-1 mAb and/or by pretreatment of EBV+BJAB cells with anti-LFA-1 mAb. Thus, we concluded that functional ICAM-1 is expressed on the HCE cells in the leading zone of the outgrowing epithelial sheet. We also could exclude the possibility that the strong positive staining at the leading edge might be caused by an artefact in immunostaining procedure. Furthermore, when the outgrowing epithelial sheet was treated with IFN-γ or TNF-α , a well known inducer of ICAM-1 expression, positive staining was observed throughout the epithelial sheet of HCE cells, suggesting that the predominant expression of ICAM-1 in the leading zone is unlikely to be caused by a certain ICAM-1 inducive factor contained possibly in the culture medium or by cytokines secreted from the cells in culture medium.
According to previous studies of epithelial cell movement, locomotory membrane activity such as ruffling was observed prominently in the cells at the leading edge of epithelial sheets by time lapse cinemicrography.21 Ruffling is thought to be an important movement corresponding to activity in the leading edge closely related to cell movement.22 Moreover, a frequent correlation between ruffling and the formation of focal adhesions is found in migrating cells.23 Focal adhesion is less adhesive in that it allows cells to move on the substrate, compared to the firm adhesion of basal epithelial cells to the underlying basal lamina mediated by the anchoring complex including hemidesmosomes.24 Focal adhesion is suggested to have a key role in the migration of cultured cells. In addition to observation in cultured cells, punctate spots of vinculin, a marker for focal adhesion, have been demonstrated in the cells at the leading edge of the migrating corneal epithelium in the healing process after epithelial debridement in a rat wound model.25 Considering our findings together with those in previous studies, it is strongly suggested that ICAM-1 is actively expressed on the epithelial cells of outgrowing epithelial sheets as an indication of active movement correlated to cell migration.
Next, to investigate the mechanism of the predominant ICAM-1 expression on the HCE cells in the leading zone of outgrowing epithelial sheets, we tried to determine the relation between PKC activity and ICAM-1 expression, because PKC has been shown to have a key role in corneal epithelial migration.14 In addition, the PKC activator, phorbor 12-myristate 13-acetate, has been reported to induce ICAM-1 expression on other types of cells such as vascular endothelial cells.26 Treatment of outgrowing epithelial cells with calphostin C, a PKC specific inhibitor, showed a markedly decreased ICAM-1 expression on the HCE cells in the leading zone, especially on the cells at the leading edge. This finding indicates that this predominant expression of ICAM-1 on the HCE cells is mediated through a PKC dependent signal transduction pathway. According to recent studies, the interaction between integrins and ECM, which occurs at sites known for focal adhesion,27 triggers an activation of protein kinases including PKC, leading to intracellular signal transduction for various cellular events.28 A transient increase of PKC activity has been demonstrated in cell membrane by cell attachment to fibronectin (FN).29 FN can be synthesised and secreted by cultured corneal epithelial cells and plays a critical part in the adhesion and the migration of corneal epithelial cells.30 Therefore, it is suggested that PKC is activated predominantly in the HCE cells in the leading zone of epithelial outgrowth by an interaction between integrins and ECM components such as FN, resulting in predominant ICAM-1 expression on these HCE cells.
Our findings indicate that ICAM-1 is expressed predominantly on the HCE cells in the leading zone, particularly at the leading edge, which is mediated through a PKC dependent pathway and possibly induced by an interaction between integrins and FN. Previous studies have shown that ICAM-1 can function as a receptor for HA which is synthesised by corneal epithelial cells in the wound healing process31,32 and enhances the migration of corneal epithelial cells cooperatively with FN in vitro.33 Taken together, ICAM-1 expression on HCE cells during active movement of the cells such as spreading and migration, possibly has an important role in an enhancement of corneal epithelial cell movement through interaction with HA, leading to acceleration of corneal epithelial wound healing cooperatively with FN.
REFERENCES
- 1.Staunton DE, Marlin SD, Stratowa C, et al. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell 1988;52:925–33. [DOI] [PubMed] [Google Scholar]
- 2.Carpén O, Pallai P, Staunton DE, et al. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and α -actinin. J Cell Biol 1992;118:1223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCourt PAG, Ek B, Forsberg N, et al. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. J Biol Chem 1994;269:30081–4. [PubMed] [Google Scholar]
- 4.Toole BP, Goldberg RL, Chi-Rosso G, et al. Hyaluronate-cell interactions. In: Trelstad, RL, ed. The role of extracellular matrix in development. New York: Liss AR, 1984:43–66.
- 5.Turley EA. The role of a cell-associated hyaluronan-binding protein in fibroblast behaviour. The biology of hyaluronan. Ciba foundation symposium 143. Chichester: Wiley, 1989:121–37. [DOI] [PubMed]
- 6.Makgoba M, Sanders ME, Show S. The CD2–LFA-3 and LFA-1–ICAM-1 pathways: relevance to T-cell recognition. Immunol Today 1989;10:417–22. [DOI] [PubMed] [Google Scholar]
- 7.Springer TA. Adhesion receptors of the immune system. Nature 1990;346:425–34. [DOI] [PubMed] [Google Scholar]
- 8.Zieske JD, Bukusoglu G, Gipson IK. Enhancement of vinculin synthesis by migrating stratified squamous epithelium. J Cell Biol 1989;109:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwata M, Sawada S, Sawa M, et al. Mechanisms of lymphocyte adhesion to cultured human corneal epithelial cells. Curr Eye Res 1997;16:751–60. [DOI] [PubMed] [Google Scholar]
- 10.Ebato B, Frend J, Thoft RA. Comparison of limbal and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci 1988;29:1533–7. [PubMed] [Google Scholar]
- 11.Iwata M, Yagihashi A, Roat MI, et al. Human leukocyte antigen-class II-positive human corneal epithelial cells activate allogeneic T cells. Invest Ophthalmol Vis Sci 1992;33:3991–4000. [PubMed] [Google Scholar]
- 12.Klein G, Sugden W, Leibold W, et al. Infection of EVB-genome negative and positive human lymphoblastoid cell lines with biologically different preparations of EVB. Intervirology 1974;3:232–4 [DOI] [PubMed] [Google Scholar]
- 13.Clements GB, Klein G, Povey S. Production by EBV infection of an EBNA-positive subline from an EBNA-negative human lymphoma cell line without detectable EBV DNA. Int J Cancer 1975;16:125–33. [DOI] [PubMed] [Google Scholar]
- 14.Lin N, Bazan HEP. Protein kinase C subspecies in rabbit corneal epithelium: increased activity of α subspecies during wound healing. Curr Eye Res 1992;11:899–907. [DOI] [PubMed] [Google Scholar]
- 15.Ofuji K, Nakamura M, Nagano T, et al. Essential role of a protein kinase C (PKC) signal transduction system in corneal epithelial migration. In: Lass JH, ed. Advances in corneal research. New York: Plenum Press, 1997:391–7.
- 16.Renkonen R, Mennander A, Ustinov J, et al. Activation of protein kinase c is crucial in the regulation of ICAM-1 expression on endothelial cells by interferon-γ. Int Immunol 1990;2:719–24. [DOI] [PubMed] [Google Scholar]
- 17.Bruns RF, Miller FD, Merriman RL, et al. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Comm 1991;176:288–93. [DOI] [PubMed] [Google Scholar]
- 18.Weiss P. The biological foundations of wound repair. In: The Harvey Lectures, series 55 (1959-1960). New York and London: Academic Press, 1961:13–42. [PubMed]
- 19.Matoltsy AG. Epidermal cells in culture. Int Rev Cytol 1960;10:315–51. [Google Scholar]
- 20.Dipasquale A, Bell Jr PB. The upper cell surface: its inability to support active cell movement in culture. J Cell Biol 1974;62:198–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughan RB, Trinkaus JP. Movement of epithelial cell sheets in vitro. J Cell Sci I 1966:407–13. [DOI] [PubMed]
- 22.Abercrombie M, Ambrose EJ. Interference microscope studies of cell contacts in tissue culture. Exp Cell Res 1985;15:332–45. [DOI] [PubMed] [Google Scholar]
- 23.Rinnerthaler G, Geiger B, Small JV. Contact formation during fibroblast locomotion: involvement of membrane ruffles and microtubules. J Cell Biol 1988;106:747–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohdadoust AA, Silverstein AM, Kenyon KR, et al. Adhesion of regenerating corneal epithelium: the role of basement membrane. Am J Ophthalmol 1968;65:339–48. [DOI] [PubMed] [Google Scholar]
- 25.Zieske JD, Bukusoglu G, Gipson IK. Enhancement of vinculin synthesis by migrating stratified squamous epithelium. J Cell Biol 1989;109: 571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voraberger G, Schafer R, Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5′-transcriptional region: induction by cytokines and phorbol ester. J Immunol 1991;147:2777–86. [PubMed] [Google Scholar]
- 27.Burridge K, Fath K, Kelly T, et al. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Ann Rev Cell Biol 1988;4;487–525. [DOI] [PubMed] [Google Scholar]
- 28.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science 1995;268:233–9. [DOI] [PubMed] [Google Scholar]
- 29.Vuori K, Ruoslahti E. Activation of protein kinase C precedes α5 β1 integrin-mediated cell spreading on fibronectin. J Biol Chem 1993;268:21459–62. [PubMed] [Google Scholar]
- 30.Ohji M, Mandarine L, Sundaraj N, et al. Corneal epithelial cell attachment with endogenous laminin and fibronectin. Invest Ophthalmol Vis Sci 1993;34:2487–92. [PubMed] [Google Scholar]
- 31.Fitzsimmons TD, Molander N, Stenevi U, et al. Endogenous hyaluronan in corneal diseases. Invest Ophthalmol Vis Sci 1994;35:2774–82. [PubMed] [Google Scholar]
- 32.Eggli P, Graber W. Ultrastructural distribution of hyaluronan in rat cornea. Exp Eye Res 1993;56:639–99. [DOI] [PubMed] [Google Scholar]
- 33.Nishida T, Nakamura M, Mishima H, et al. Hyaluronan stimulates corneal epithelial migration. Exp Eye Res 1991;53:753–8. [DOI] [PubMed] [Google Scholar]