Abstract
Aims: To determine the results of pars plana vitrectomy (PPV) and silicone oil infusion (SOI) in severe proliferative diabetic retinopathy (PDR).
Methods: The records of 23 eyes (21 patients: 12 males, nine females) with PDR who had undergone PPV and SOI were reviewed retrospectively.
Results: Average follow up was 5.4 months (range 1–25). Surgical indications were tractional retinal detachment (TRD) (17.4%), traction-rhegmatogenous retinal detachment (TRRD) (8.7%), TRD with vitreous haemorrhage (VH) (48%), TRD with neovascular glaucoma (NVG) (8.6%), TRD with fibrinoid syndrome (FS) (17.3%). With one operation, the retinal reattachment rate was 17/23 (74%). Among these 23 eyes, 11 (48%) had previously failed vitrectomy, and the retina was attached in 8/11 (73%) with a single procedure. With additional surgery employing PPV and SOI, the final reattachment rate was 20/23 (87%). The only cases with intraocular pressure <5 mm Hg had retinal detachment. Postoperative visual acuity (VA) improved in 10 eyes (44%), was unchanged in three (12%), and decreased in 10 eyes (44%).
Conclusion: SO tamponade is useful in severely diseased eyes with PDR, even in the presence of rubeosis iridis (RI) and NVG, FS, or in cases with previously failed vitrectomy, especially in the presence of RI.
Keywords: diabetes, silicone oil, proliferative diabetic retinopathy
Despite the success of pars plana vitrectomy in managing the severe complications of diabetic retinopathy, significant operative and postoperative complications still occur leading to anatomical failure and blindness. Recurrence of retinal detachment secondary to fibrovascular proliferation, progression of neovascularisation with neovascular glaucoma, hypotony with subsequent phthisis bulbi, and fibrinoid syndrome are some of the many reported postoperative complications of diabetic vitrectomy.
Silicone oil facilitates retinal reattachment by providing extended intraocular tamponade. Silicone oil may compartmentalise the eye1 and may have a role in inhibiting progressive neovascularisation in the anterior segment by preventing the diffusion of angiogenic substances. In addition, silicone oil can prevent hypotony and subsequent phthisis bulbi.2
We reviewed results of pars plana vitrectomy (PPV) in eyes with severe proliferative diabetic retinopathy (PDR) that underwent silicone oil infusion (SOI). The results indicate that PPV with SOI can salvage useful vision in eyes with an otherwise poor prognosis.
MATERIALS AND METHODS
We retrospectively reviewed the data for 21 patients who underwent standard three port pars plana vitrectomy and SOI for PDR at University Hospital, Newark, NJ, USA, between 1997 and 2002. All patients underwent complete preoperative and postoperative examinations including visual acuity testing, slit lamp biomicroscopy, gonioscopy, intraocular pressure (IOP) measurement, indirect ophthalmoscopy, and ultrasonography when the fundus could not be visualised. To compare cases of similar complexity, a “complexity score” (CS) was defined. The complexity score was graded by quantifying: (1) the number of quadrants of fibrovascular proliferation (FVP, 1–4 quadrants, each quadrant involved corresponds to 1 point increase in the CS); (2) the location of FVP: anterior to the equator (0 points), posterior to the equator (0 points), both anterior and posterior (1 point); (3) tractional retinal detachment (TRD, 1 point); (4) traction-rhegmatogenous retinal detachment (TRRD, 2 points); (5) the presence or absence of a posterior vitreous detachment (no PVD, 1 point). Anatomical success was defined as complete attachment of the retina or attachment posterior to the scleral buckle, if present.
RESULTS (TABLE 1)
Table 1.
Functional and anatomical status after vitrectomy with silicone oil infusion for proliferative diabetic vitrectomy. Patients 7, 16, and 23 had repeat vitrectomy and silicone oil infusion with final retinal reattachment
| Case No | Age/sex/ race/eye | Diagnosis | Length of follow up (months) | Vision (preop/final) | IOP (preop/postop) | Rubeosis (preop/postop) | NVG (preop/postop) | Anatomical outcome |
| 1 | 58/F/H/RE | PDR, TRRD, RI, hypotony | 1 | LP/LP | 6 mm/5 mm | RI/no change | None | Retina flat SO present |
| 2 | 59/F/AA/RE | PDR, TRD, VH | 5 | HM/CF 1 ft | 19 mm/22 mm | None | None | Retina flat SO present |
| 3 | 70/M/AA/LE | PDR, TRD, recurrent VH | 4 | CF 2ft/ 5/200 |
22 mm/15 mm | None/RI | None | Retina flat SO present |
| 4 | 21/F/AA/RE | PDR, RI, NVG, TRD, subluxed lens | 12 | CF 2ft/ 5/160 | 16 mm/7 mm | RI/regressed | NVG/resolved | Retina flat SO present |
| 5 | 21/F/AA/LE | PDR, RI NVG, TRD, FS | 1 | LP/LP | 14 mm/20 mm | RI/no change | NVG/IOP is normal | Retina flat SO present |
| 6 | 60/M/AA/RE | PDR, TRD, RI, FS | 4 | LP/10/200 | 12 mm/9 mm | RI/regressed | None | Retina flat SO present |
| 7 | 52/M/H/RE | PDR, TRD, VH, FS | 12 | LP/HM | 9 mm/2 mm | None | None | Recurrent RD SO present |
| 8 | 44/M/H/RE | PDR, TRD, VH | 2 | CF 1 ft/ 20/80 |
10 mm/15 mm | None | None | Retina flat SO removed |
| 9 | 36/M/AA/RE | PDR, TRD, VH | 1 | CF 2 ft/ 5/200 |
17 mm/21 mm | None | None | Retina flat SO present |
| 10 | 36/M/AA/LE | PDR, TRD, VH | 3 | HM/LP | 15 mm/14 mm | None | None | Retina flat SO present |
| 11 | 49/F/AA/RE | PDR, TRD, VH, CT | 1 | CF 1 ft/HM | 11 mm/32 mm | None | None | RD |
| 12 | 82/F/AA/RE | PDR, TRD | 3 | LP/NLP | 14 mm/8 mm | None | None | Retina flat |
| 13 | 52/M/AA/RE | PDR, TRD, RI | 9 | HM/LP | 12 mm/2 mm | RI/regressed | None | RD SO present |
| 14 | 75/F/AA/RE | PDR, TRD, VH | 18 | LP/HM | 9 mm/3 mm | None | None | RD (peripheral) SO present |
| 15 | 52/F/H/LE | PDR, TRD, CT, RI HYP | 1 | HM/CF 1 ft | 12 mm/12 mm | RI/no change | None | Retina flat SO present |
| 16 | 68/M/AA/LE | PDR, TRRD | 3 | CF 2 ft/HM | 19 mm/15 mm | None | None | TRD |
| 17 | 58/M/AA/RE | PDR, TRD, VH | 1 | CF 1 ft/LP | 16 mm/15 mm | None | None | Retina flat SO present |
| 18 | 29/F/H/LE | PDR, TRD, VH | 6 | HM/LP | 13 mm/12 mm | None | None | RD SO present |
| 19 | 59/F/AA/RE | PDR, TRD, ERM, VH | 25 | 5/200/ CF 2 ft |
12 mm/27 mm | None | None | Retina flat SO present |
| 20 | 58/M/AA/RE | PDR, TRD, FS | 8 | LP/CF 2 ft | 13 mm/16 mm | None | None | Retina flat SO removed |
| 21 | 74/M/AA/RE | PDR, TRD, CSME | 2 | HM/HM | 10 mm/25 mm | None | None | Retina flat SO removed |
| 22 | 56/M/H/LE | PDR, TRD, VH, RI, NVG | 1 | LP/NLP | 32 mm/5 mm | RI/no change | NVG/IOP is normal | Retina flat SO present |
| 23 | 58/M/H/LE | PDR, TRD, VH | 2 | HM/LP | 14 mm/22 mm | None/RI | None | RD SO present |
PDR = proliferative diabetic retinopathy; TRRD = tractional rhegmatogenous retinal detachment; RI = rubeosis iridis; SO = silicone oil; TRD = tractional retinal detachment; VH = vitreous haemorrhage; NVG = neovascular glaucoma; FS = fibrinoid syndrome; RD = retinal detachment; CT = cataract; HYP = hyphaema; ERM = epiretinal membrane.
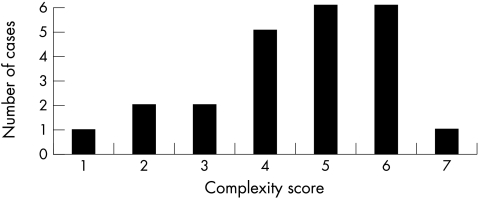
The study comprised 23 eyes (21 patients): 12 males (57%) and nine females (43%); seven Hispanics (33%); 14 (67%) African-Americans. The average age was 53.6 years (range 21–82 years). Surgical indications were: tractional retinal detachment (TRD) (four eyes, 17.4%), traction-rhegmatogenous retinal detachment (TRRD) (two eyes, 8.7%), TRD with vitreous haemorrhage (VH) (11 eyes, 48%), TRD with VH and neovascular glaucoma (NVG) (one eye, 4.3%), TRD with NVG (one eye, 4.3%), TRD with fibrinoid syndrome (FS) (two eyes, 8.7%), TRD with FS and NVG (one eye, 4.3%) and TRD with VH and FS (one eye, 4.3%). The average complexity score was 4.5 (Fig 1). The length of follow up ranged from 1–25 months (mean 5.4 months).
Figure 1.
Distribution of cases by complexity score.
In 12 eyes (52%), SOI was part of the initial operation during primary vitrectomy. In 11 eyes (48%), SOI was done after previous failed vitrectomy. Preoperatively, 14 patients were phakic, six were pseudophakic, and three were aphakic. Eight of 14 (57%) phakic eyes underwent lensectomy (five were left aphakic). Four of the pseudophakic eyes underwent intraocular lens removal and were left aphakic. Different intraocular procedures were performed at the time of surgery. Scleral buckles were placed in two (8.7%) eyes. Epiciliary dissection was done in two (8.7%) eyes, one of which had preoperative hypotony. Cyclocryotherapy was done intraoperatively in two (8.7%) eyes. Membrane peeling was done in all eyes. A Baerveldt valve was placed in one (4.3%) eye, and a peripheral relaxing retinectomy was necessary in one (4.3%) eye. SO was removed in two (8.7%) eyes at 5 and 8 months, respectively, without complication. Silicone was removed because of migration into the anterior segment with corneal touch in one eye and cataract progression in the second eye. In one eye, the oil was removed because it was present in the anterior chamber.
After a single operation employing PPV and SOI, anatomical attachment was achieved in 17 (74%) of 23 eyes. In the 12 eyes that were operated for the first time with primary SOI, the retina was attached in nine (75%). In the 11 eyes that underwent SOI after previously failed vitrectomy, the retina was attached in eight (73%). The difference in the reattachment rate between these two groups was not statistically significant (χ2 test, p= 0.9). Three of the six eyes with recurrent detachment underwent repeat vitrectomy, membrane peeling, and silicone insertion with successful reattachment. (The remaining three patients declined additional surgery.) Therefore, with multiple operations employing silicone oil insertion, the final anatomical success rate at last follow up was 20/23 (87%). The length of follow up after the last procedure for the three reoperated eyes ranged from 3 to 4 months (mean 3.3 months).
Case complexity did not correlate with anatomical success (Table 2).
Table 2.
Case complexity v surgical outcome
| Anatomical outcome | ||
| Complexity score | Flat | Retinal detachment |
| 1 | 1 | 0 |
| 2 | 1 | 1 |
| 3 | 2 | 0 |
| 4 | 4 | 1 |
| 5 | 3 | 3 |
| 6 | 6 | 0 |
| 7 | 0 | 1 |
Postoperative visual acuity (VA) improved in 10 eyes (44%), was unchanged in three (12%) and decreased in 10 eyes (44%). Two eyes (8.7%) had no light perception at last follow up (neither underwent scleral buckling). Both eyes had preoperative vision of light perception. Loss of light perception was due to retinal and optic nerve ischaemia in each case. Preoperatively, the visual acuity of all patients ranged from light perception to less than counting fingers at 1 foot except for one patient whose vision was 5/200. After surgery, five of 23 eyes (22%), had vision ≥5/200.
Rubeosis iridis (RI) was present preoperatively in seven eyes (30.4%), three of which had NVG. Postoperatively, three of seven eyes had regressed rubeosis. Of the three eyes with NVG, one underwent Baerveldt valve placement with normalisation of the postoperative intraocular pressure. In another, the NVG regressed after surgery. The last eye had regression of neovascular glaucoma but no light perception postoperatively because of ischaemia. Only one eye developed de novo RI postoperatively. Postoperatively, at last follow up, the intraocular pressure was > 29 mm Hg in only one patient, who did not have neovascular glaucoma. This patient was using antiglaucoma medications preoperatively. At the last follow up visit after surgery, five eyes (23%) had intraocular pressure ≤5 mm Hg. Three of these eyes had persistent retinal detachment, which we presume to be the cause of the hypotony (Table 1). The remaining 17 eyes had intraocular pressure ranging from 6–29 mm Hg, with two patients taking antiglaucoma medications.
Intraoperative complications included iatrogenic retinal breaks during membrane peeling in four of 23 eyes (17.3%). Postoperative complications included keratopathy (two eyes), SO in the anterior chamber (one eye), cataract (one eye), fibrinoid reaction with pupillary membrane formation (one eye), and new onset of RI (one eye). One eye with preoperative fibrinoid syndrome had persistent fibrinoid reaction postoperatively and underwent intraocular tissue plasminogen activator injection at the slit lamp with clinical improvement.
DISCUSSION
Silicone oil promotes retinal reattachment by providing extended intraocular tamponade. Intraocular gas has greater surface tension and spontaneously reabsorbs, but its effect is shorter and does not always create an anatomically stable situation. Silicone oil has been advocated for the treatment of complex retinal detachment3–8 and treatment of severe PDR.9–13
Previously, other authors have reported clinical series in which silicone oil infusion was used in the management of severe PDR. Lean et al12 reported a series of 13 eyes that underwent surgery for severe diabetic traction detachment. Four eyes (31%) remained attached with visual improvement in one (7.7%).
Rinkoff and de Juan10 reported on 10 patients with rhegmatogenous retinal detachment complicated by advanced proliferative vitreoretinopathy following failed vitrectomy for PDR. In this series, three eyes (30%) achieved retinal reattachment. Yeo et al14 reported a series of 23 eyes with PDR in which retinal reattachment was achieved in 16 (70%), and final vision of 5/200 or better was obtained in five eyes (22%). Lucke et al9 reported a series of 106 patients with PDR, and the reattachment rate was 73%. Brourman et al13 reported a series of 34 patients (37 eyes) with refractory severe neovascular glaucoma and/or recurrent retinal detachment from PDR. Reattachment was maintained in 26 eyes (70%). Regression of iris neovascularisation occurred in eight (36%) of 22 eyes.
In a more recent prospective study, Azen et al15 reported a series of 340 patients (359 eyes) with PDR that underwent vitrectomy and silicone oil infusion. They found the frequency of complete retinal reattachment at the last follow up was 57% whereas the rate of macular reattachment was 74%. In their series, vision ≥4/200 was retained in 24% of eyes.
In a prospective multicentre study, Scott et al16 reviewed the results of patients who underwent vitrectomy with silicone oil infusion for retinal detachment associated with cytomegalovirus retinitis or non-cytomegalovirus necrotising retinitis, including proliferative diabetic retinopathy, giant retinal tear, proliferative vitreoretinopathy, and ocular trauma. In their series, 127 patients (132 eyes) underwent vitrectomy and silicone oil infusion for PDR. The reattachment rate of eyes undergoing the first operation or after previously failed vitrectomy was 56/116 (48%) and 8/16 (50%), respectively.
In our study, we found no difference in the reattachment rate among eyes undergoing first time vitrectomy (75%) versus those that had previously failed vitrectomy (73%) (χ2 test, p= 0.9). All three eyes that underwent PPV and SOI twice were reattached after the second procedure. Therefore, with multiple operations employing PPV and SOI, the final anatomical success rate was 20/23 (87%).
Among the 17 eyes with a flat retina, nine (53%) had some degree of visual improvement after surgery. Only five eyes (29% of anatomically successful cases and 22% of all cases) recovered ambulatory vision (that is, ≥5/200) at last follow up. This result probably reflects, in part, the fact that preoperative visual acuity was poor in most eyes. At last follow up, two eyes (8.7%) had no light perception. One of the two developed the fibrinoid syndrome after surgery and had a flat retina. The other patient had preoperative neovascular glaucoma with a flat retina at last follow up.
In the series reported by Rinkoff and de Juan10 two (20%) of 10 patients regained ambulatory vision. Yeo et al14 reported final vision of 5/200 or better in five (22%) of 23 eyes. Lucke et al9 reported visual acuity of 5/200 or better in 65% of patients. Brourman et al13 reported final visual acuity of at least 5/200 in nine (24%) of 37 eyes and nine (35%) of 26 anatomically successful cases. Azen et al15 reported vision ≥4/200 in 24% of eyes. Scott et al16 reported a final ambulatory vision in 45% of the reported eyes. In their series, a higher rate of ambulatory vision was also achieved for primary anatomical success cases compared to reoperated cases (48% v 25%) although differences were not statistically significant (p=0.1).
Rubeosis iridis was present preoperatively in seven eyes, three of which had neovascular glaucoma. Rubeosis regressed in three (43%) of the seven eyes, and none of the patients had active neovascular glaucoma at last follow up. Regression of neovascularisation after silicone oil infusion has been reported in other series. Bourman et al reported regression in 36% of eyes with rubeosis iridis.13
The rationale for using silicone oil in the management of anterior segment neovascularisation is that silicone compartmentalises the eye and confines angiogenic substances to the posterior segment.1,17 In addition, silicone oil acts as a diffusion/convection barrier to oxygen, as it prevents the decrease in anterior chamber oxygen tension that occurs after lensectomy and vitrectomy in cats.18 Silicone oil does not always prevent the development of neovascularisation.10,14 In our series, one eye developed de novo rubeosis iridis, and another eye developed increased posterior segment neovascularisation. Peri-silicone proliferation may be due to entrapment of retina derived angiogenic substances between the retina surface and the silicone oil bubble.19
Hypotony in this setting can result from cyclitic membrane formation with ciliary body detachment and/or sequestration or from ciliary body atrophy.20,21 The high percentage of eyes with hypotony (IOP = 5 mm Hg) at last follow up (5/23 (22%)) has been noted by others. Azen et al15 reported an incidence of 21% among 359 eyes. Brourman et al13 reported an incidence of 22% in 37 eyes. In contrast, Yeo et al14 reported no cases of hypotony in a series of 23 eyes. All the eyes with postoperative hypotony in our series had undergone multiple vitrectomies before silicone oil infusion.
Four eyes in this series had the fibrinoid syndrome preoperatively, most likely secondary to excessive postoperative inflammation following previous failed vitrectomy and laser photocoagulation. Schepens described the fibrinoid syndrome.22 It is characterised by fibrin strand formation in the vitreous cavity, condensation of fibrinoid material on the surface of the retina, eventual development of turbid vitreous gel (or fluid, in eyes that have undergone vitrectomy) and, often, in progressive traction retinal detachment. Other features include neovascular glaucoma, pupillary block glaucoma, recurrent haemorrhage, cataract, and ciliary body detachment with hypotony. The incidence of fibrinoid syndrome in patients undergoing vitrectomy for the complications of PDR is approximately 8%.23 In one series, postvitrectomy fibrin formation occurred in 49 of 222 eyes (22%) within 72 hours of surgery.24 The prognosis is generally poor. In the present series, of the four eyes with preoperative fibrinoid syndrome, vision improved in three eyes (one from light perception to counting fingers, another from light perception to hand movements, and the last from light perception to 10/200). In the remaining eye, the vision was unchanged at light perception. One of the patients had persistent fibrinoid syndrome and therefore underwent intravitreal tissue plasminogen activator injection (50 μg/0.1 ml) at the slit lamp with resolution of the fibrin strands. We believe that aggressive management including excision of the posterior hyaloid face and vitreous base dissection, extensive panretinal photocoagulation (to induce regression of or prevent anterior hyaloidal fibrovascular proliferation), and silicone oil infusion can create a stable anatomical situation even in eyes with the fibrinoid syndrome.
One patient developed de novo postoperative fibrinoid syndrome after PPV with SOI. Other authors reported fibrin formation following vitrectomy and silicone oil infusion.25 Low molecular weight components and other impurities in the silicone oil may cause a postoperative inflammatory reaction. The eye that developed de novo fibrinoid syndrome postoperatively had a flat retina at last follow up with no light perception vision.
There was no correlation between case complexity and anatomical success. In a retrospective study comparing the use of viscodelamination versus en bloc dissection, we reported 150 eyes with PDR that underwent pars plana vitrectomy with membrane peeling.26 To compare cases of similar anatomical complexity, the same “complexity score” (CS) was employed. The reattachment rate in that series among eyes with 6 months of follow up was 36/43 (84%) among cases undergoing viscodissection v 55/58 (95%) without viscodissection. In that series, the average complexity score was 3.7, statistically significantly lower than the average complexity score of this series (p=0.03). We were not able to compare the results with eyes of a given complexity score in that study with those of similarly complex eyes in the present study because the sample of eyes with silicone infusion is too small.
The outcome of this study further supports the role of silicone oil infusion as a useful option in severely diseased eyes with PDR, even in the presence of rubeosis iridis, neovascular glaucoma, or the fibrinoid syndrome. In the group of eyes that underwent pars plana vitrectomy and silicone infusion at the time of the first vitrectomy, the retinal reattachment rate was 75%, and no eye developed hypotony after surgery.
Acknowledgments
Supported by grants from Research to Prevent Blindness, Inc, the Eye Institute of New Jersey, and the New Jersey Lions Eye Research Foundation.
Presented in part at the Association for Research in Vision and Ophthalmology annual meeting, Fort Lauderdale, Florida, 2002.
The authors acknowledge Pamela D’Amato, MD for assistance in manuscript preparation.
REFERENCES
- 1.Charles S. Vitreous surgery. 2nd ed. Baltimore: Williams and Wilkins, 1987:122.
- 2.Morse LS, McCuen BW 2nd. The use of silicone oil in uveitis and hypotony. Retina 1991;11:399–404. [DOI] [PubMed] [Google Scholar]
- 3.Grey RH, Leaver PK. Results of silicone oil injection in massive preretinal retraction. Trans Ophthalmol Soc UK 1977;97:238–41. [PubMed] [Google Scholar]
- 4.Grey RH, Leaver PK. Silicone oil in the treatment of massive preretinal retraction. I. Results in 105 eyes. Br J Ophthalmol 1979;63:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCuen BW 2nd, Landers MB 3rd, Machemer R. The use of silicone oil following failed vitrectomy for retinal detachment with advanced proliferative vitreoretinopathy. Ophthalmology 1985;92:1029–34. [DOI] [PubMed] [Google Scholar]
- 6.Sell CH, McCuen BW 2nd, Landers MB 3rd, et al. Long-term results of successful vitrectomy with silicone oil for advanced proliferative vitreoretinopathy. Am J Ophthalmol 1987;103:24–8. [DOI] [PubMed] [Google Scholar]
- 7.Aaberg TM. Management of anterior and posterior proliferative vitreoretinopathy. XLV. Edward Jackson memorial lecture. Am J Ophthalmol 1988;106:519–32. [DOI] [PubMed] [Google Scholar]
- 8.Cox MS, Trese MT, Murphy PL. Silicone oil for advanced proliferative vitreoretinopathy. Ophthalmology 1986;93:646–50. [DOI] [PubMed] [Google Scholar]
- 9.Lucke KH, Foerster MH, Laqua H. Long-term results of vitrectomy and silicone oil in 500 cases of complicated retinal detachments. Am J Ophthalmol 1987;104:624–33. [DOI] [PubMed] [Google Scholar]
- 10.Rinkoff JS, de Juan E Jr, McCuen BW 2nd. Silicone oil for retinal detachment with advanced proliferative vitreoretinopathy following failed vitrectomy for proliferative diabetic retinopathy. Am J Ophthalmol 1986;101:181–6. [DOI] [PubMed] [Google Scholar]
- 11.McCuen BW 2nd, Rinkoff JS. Silicone oil for progressive anterior ocular neovascularization after failed diabetic vitrectomy. Arch Ophthalmol 1989;107:677–82. [DOI] [PubMed] [Google Scholar]
- 12.Lean JS, Leaver PK, Cooling RJ, et al. Management of complex retinal detachments by vitrectomy and fluid/silicone exchange. Trans Ophthalmol Soc UK 1982;102:203–5. [PubMed] [Google Scholar]
- 13.Brourman ND, Blumenkranz MS, Cox MS, et al. Silicone oil for the treatment of severe proliferative diabetic retinopathy. Ophthalmology 1989;96:759–64. [DOI] [PubMed] [Google Scholar]
- 14.Yeo JH, Glaser BM, Michels RG. Silicone oil in the treatment of complicated retinal detachments. Ophthalmology 1987;94:1109–13. [DOI] [PubMed] [Google Scholar]
- 15.Azen SP, Scott IU, Flynn HW Jr, et al. Silicone oil in the repair of complex retinal detachments. A prospective observational multicenter study. Ophthalmology 1998;105:1587–97. [DOI] [PubMed] [Google Scholar]
- 16.Scott IU, Flynn HW, Lai M, et al. First operation anatomic success and other predictors of postoperative vision after complex retinal detachment repair with vitrectomy and silicone oil tamponade. Am J Ophthalmol 2000;130:745–50. [DOI] [PubMed] [Google Scholar]
- 17.Ashton N. Retinal neovascularization in health and disease. Am J Ophthalmol 1957;44:7–17. [DOI] [PubMed] [Google Scholar]
- 18.De Juan E Jr, Hardy M, Hatchell DL, et al. The effect of intraocular silicone oil on anterior chamber oxygen pressure in cats. Arch Ophthalmol 1986;104:1063–4. [DOI] [PubMed] [Google Scholar]
- 19.Glaser BM, D’Amore PA, Michels RG, et al. Demonstration of vasoproliferative activity from mammalian retina. J Cell Biol 1980;84:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugar HS, Okamura ID. Ocular findings six years after intravitreal silicone injection. Arch Ophthalmol 1976;94:612–5. [DOI] [PubMed] [Google Scholar]
- 21.Fegan R, Del Priore, Kim E, et al. Does viscodissection result in less iatrogenic retinal breaks during membrane peeling of diabetic fibrovascular proliferative membranes? Invest Ophthalmol Vis Sci 2001;42:S104. [Google Scholar]
- 22.Schepens CL. Clinical and research aspects of subtotal open-sky vitrectomy. XXXVII Edward Jackson Memorial Lecture. Am J Ophthalmol 1981;91:143–71. [DOI] [PubMed] [Google Scholar]
- 23.Lewis H, Abrams GW, Williams GA. Anterior hyaloidal fibrovascular proliferation after diabetic vitrectomy. Am J Ophthalmol 1987;104:607–13. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe GJ, Schwartz D, Han DP, et al. Risk factors for postvitrectomy fibrin formation. Am J Ophthalmol 1990;109:661–7. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RN, Flynn HW Jr, Parel JM, et al. Transient hypopyon with marked anterior chamber fibrin following pars plana vitrectomy and silicone oil injection. Arch Ophthalmol 1989;107:683–6. [DOI] [PubMed] [Google Scholar]
- 26.Zarbin MA, Michels RG, Green WR. Dissection of epiciliary tissue to treat chronic hypotony after surgery for retinal detachment with proliferative vitreoretinopathy. Retina 1991;11:208–13. [DOI] [PubMed] [Google Scholar]