Abstract
Background/aims: Hyaluronan is present in the trabecular meshwork where it is involved in the pathophysiology of aqueous outflow environment. In this study, the expression and regulation of hyaluronan synthase (HAS), which is the enzyme synthesising hyaluronan, in trabecular meshwork cells were investigated.
Methods: Cultured bovine trabecular meshwork cells (BTMCs) were used. HAS expression in BTMCs was examined by RT-PCR. The effects of transforming growth factor β (TGF-β) and platelet derived growth factor BB (PDGF-BB) on HAS expression in BTMCs were examined by quantitative RT-PCR. The HAS2 expression by TGF-β and PDGF-BB at the protein level was also confirmed immunohistochemically. The production of hyaluronan from BTMCs was detected by high performance liquid chromatography (HPLC).
Results: Three HAS isoforms were expressed in BTMCs at the mRNA level. Among HAS isoforms, only the expression of HAS2 mRNA was increased by the administration of TGF-β or PDGF-BB. HAS2 upregulation by these growth factors was also confirmed at the protein level. Further, hyaluronan production from BTMCs was stimulated by TGF-β or PDGF-BB.
Conclusion: Expression of HAS in trabecular meshwork may maintain the hyaluronan content in the aqueous outflow pathway. Its production is regulated by TGF-β and PDGF-BB. The regulation of the expression of HAS in trabecular meshwork might be useful for modulating the aqueous outflow environment.
Hyaluronan, a non-sulfated glycosaminoglycan (GAG), is composed of β1,4 linked repeating disaccharides of d-glucuronic acid β1,3 linked to N-acetylglucosamine-β1,4.1 This molecule is an important constituent of the extracellular matrix (ECM) and plays an essential part in regulating biological dynamic status of cell migration, proliferation, adhesion, development, and differentiation.2,3 Hyaluronan is synthesised at the inner surface of the plasma membrane by three related isoenzymes, hyaluronan synthases (HAS1, HAS2, HAS3).4 Each HAS isoform possesses the ability to synthesise hyaluronan molecules of a given size, exhibit different kinetic properties, and a cell type specific pattern.4–6 The expression levels of each HAS gene and protein are regulated by several cytokines and growth factors, such as platelet derived growth factor BB (PDGF-BB) and transforming growth factor β (TGF-β).7–9
GAG expression has been identified in trabecular meshwork (TM) in several kinds of eyes.10–13 Among the GAGs of TM12 hyaluronan is the most abundant and has been suggested to be of importance in the regulation of aqueous outflow.10 Further, it is suggested that the alteration of hyaluronan in TM might be involved in pathophysiology of primary open angle glaucoma.14 We and another group previously showed that HAS is expressed in the ocular tissue, including TM.15,16 In corneal endothelial cells, expression of HAS was regulated by TGF-β through Smad protein,9 which has been identified as an intracellular signalling molecule downstream of TGF-β type I receptor.17 Furthermore, it has been shown that TM cells express both TGF-βs and their receptors,18,19 and also secrete the cytokines. However, the regulation of hyaluronan and HAS in TM have remained unclear yet.
In the present study, we investigated the regulation of hyaluronan and HAS in response to growth factors in cultured TM cells to gain further insight into the mechanism of aqueous outflow modulation.
MATERIALS AND METHODS
Cell culture
Cultured bovine trabecular meshwork cells (BTMCs) were used in this study and were prepared according to methods reported previously.20 A brief summary of the process is as follows. Three year old bovine eyes were obtained from a slaughterhouse. Under a microscope, trabecular tissue was dissected carefully and placed on a six well culture plate (Falcon, Lincoln Park, NJ, USA) in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) with 15% fetal bovine serum (FBS) and 20 μg/ml gentamicin (Gibco) in a humidified atmosphere at 37°C in 5% CO2. BTMCs migrated from the tissue explant and were treated with trypsin-EDTA (Gibco). Cultures of BTMCs in DMEM medium, between the second and fourth passage (P2∼P4), were used in this study.
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR was performed using a cDNA template derived from mRNA of BTMCs according to methods reported previously.9,16 Briefly, total RNA was isolated using Isogen (Nippon Gene, Toyama, Japan) from cells and cDNA was made using Super Script II (Gibco, Grand Island, NY, USA) as a reverse transcriptase. PCR conditions have been described previously.9 The amplified fragments obtained from RT-PCR were also subcloned into TA vectors (Invitrogen, San Diego, CA, USA) and sequenced according to the manufacturer’s protocols for further confirmation of identification.
Competitive RT-PCR
To investigate the regulation of HAS expression at the mRNA level in response to external stimuli, recombinant human TGF-β1 or PDGF-BB (R&D, Minneapolis, MN, USA) at 10 ng/ml were added to quiescent BTMCs in DMEM containing 1% FBS. After 12 hours of incubation at 37°C in 5% CO2, the cells were harvested for quantitative PCR. The competitive PCR was performed using LightCycler (Roche Diagnostics GmbH, Mannheim, Germany) with specific fluorescein hybridisation probes. PCR conditions and methods of calculation of the results have been described previously.9
Immunocytochemistry
The visualisation of HAS2 at the protein level in response to TGF-β and PDGF-BB stimulation in BTMCs was detected immunocytochemically. Cells on glass slides were cultured in the absence or presence of 10 ng/ml TGF-β1 or PDGF-BB for 48 hours after 24 hours of starvation in DMEM containing 1% FBS. Thereafter, the cells were washed with phosphate buffered saline (PBS) twice and fixed with 70% ethanol for 10 minutes at 4°C. After washing with PBS, the samples were treated with non-immune goat serum for 20 minutes at room temperature to block non-specific binding of the antibodies. Then the cells were incubated with the affinity purified anti-human HAS2 polyclonal antibody8 as the primary antibody at 10 μg/ml at room temperature for 1 hour. For the negative control, non-immune goat serum IgG (Vector Laboratories, Burlingame, CA, USA) was used in place of the primary antibody. Immunoreactivity was detected with a Histofine SAB-PO Kit (Nichirei Corporation, Tokyo, Japan) according to the manufacturer’s protocol. The final reaction product was visualised with 3,3’-diaminobenzidine tetrahydrochloride (DAB) for 5 minutes.
Quantitative analysis of HAS2 immunohistochemistry was determined using Photoshop based image analysis.21 Briefly, three ×40 fields of each group (no treatment, TGF-β1 treatment, and PDGF-BB treatment) were chosen and were analysed by the Photoshop, version 3.0 (Adobe Systems, Mountain View, CA, USA). Immunostained cells were transformed to grey scale and optical density plot of selected area was generated using Histogram tool. Immunostaining intensity (arbitrary unit, AU) was calculated as the difference between membrane or cytoplasm immunostaining and background and was normalised by pixel area.
Determination of hyaluronan content in the culture media
The effect of TGF-β or PDGF-BB on hyaluronan production in BTMCs was observed by high performance liquid chromatography (HPLC) according to the previous report by Miyauchi et al.22 Briefly, the media (200 μl) were incubated with 200 mU of chondroitinase (Chase) ABC (Seikagaku Corporation, Tokyo, Japan) for 2 hours at 37°C. The digested samples were mixed with 200 mU of chondroitinase AC-II solution (Seikagaku Corporation) and 40 μl of 1M sodium acetate buffer (pH 6.0), and incubated for an additional 2 hours at 37°C. Then the samples were centrifuged for 10 minutes at 3000 g and supernatants were ultrafiltered using an Ultrafree C3GC (cut-off molecular size 10 000, Japan Millipore Limited, Tokyo, Japan). Unsaturated disaccharides derived from hyaluronan (ΔDi-hyaluronan) and chondroitin sulfate (ΔDi-0S, ΔDi-4S, ΔDi-6S) were recovered in the filtrate solution and their identities were determined using HPLC.
RESULTS
Effects of TGF-β and PDGF-BB on HAS gene expression in TM cells
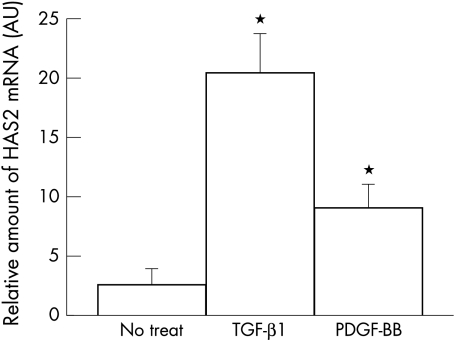
The expressions of each HAS isoform in BTMCs at the mRNA level were examined by RT-PCR. The transcripts detected by each set of the primers for HAS1, HAS2, and HAS3 had sizes of 533 bp, 609 bp, and 585 bp, respectively (data not shown). The sequences determined from each amplified cDNA fragment were completely identical to those of the corresponding portions of bovine HAS isoforms. Quantification of the effects of growth factors on HAS expression in BTMCs was performed by real time quantitative RT-PCR. Stimulation of the cells with TGF-β induced about a sevenfold upregulation of HAS2 mRNA compared with the non-treated cells (n=3, p<0.05, Fig 1). Treatment of the cells with PDGF-BB led to about a threefold increase in HAS2 mRNA over the levels of non-stimulated cells (n=3, p<0.05, Fig 1). The expression levels of HAS1 or HAS3 were lower than that of HAS2 in BTMCs and induction of their transcripts in response to growth factors was not detected (data not shown).
Figure 1.
Detection of HAS2 mRNA in response to external stimuli. HAS2 mRNA expression in response to 10 ng/ml of TGF-β1 or PDGF-BB in BTMCs was analysed by competitive PCR. Real time fluorescein PCR detection (LightCycler) was used in this experiment (n = 6, error bar is SD). The y-axis gives standardising log concentration values, which are obtained from a standard curve, and show the relative amount of HAS2 mRNA.
Effects of TGF-β and PDGF-BB on HAS2 protein expression
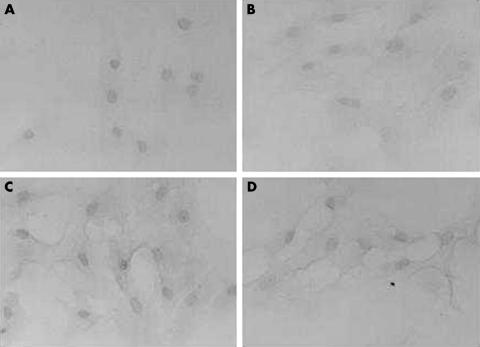
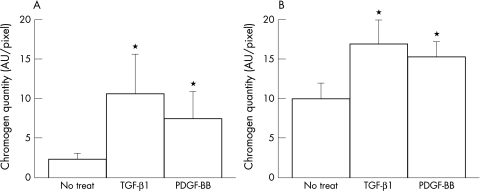
To investigate whether the stimulation of HAS2 mRNA in BTMCs in response to TGF-β1 or PDGF-BB also led to an increase in the protein levels of HAS2, we examined its expression using an antiserum against the C-terminal of mouse HAS2 by semiquantitative immunohistochemistry. Cultured BTMCs without growth factor stimulation were stained positively both in plasma membrane and partial cytoplasm by anti-HAS2 antibody (Fig 2B), suggesting that BTMCs constitutively express HAS2 protein. However, after growth factor stimulation, HAS2 immunoreactivity was considerably stronger compared to untreated cultures, particularly in plasma membrane of BTMCs (Fig 2C, D). This was further confirmed by densitometric analysis; TGF-β1 (Fig 3A) or PDGF-BB (Fig 3B) treatment resulted in an increased chromogen index on the membrane portion. Thus, it is possible that TGF-β and PDGF-BB induce HAS2 protein translocalisation from cytoplasm to membrane.
Figure 2.
Immunohistochemical detection of HAS2 in BTMCs. Ethanol fixed BTMCs were incubated in the absence or presence of anti-HAS2 antibody and visualised by DAB chromogen. (A) Control IgG (isotype control), (B) no treatment, (C) treatment with 10 ng/ml of TGF-β1, (D) treatment with 10 ng/ml of PDGF-BB. BTMCs were weakly stained by anti-HAS2 polyclonal antibody in plasma membrane and partial cytoplasm without TGF-β stimulation (B). When BTMCs were incubated with growth factors, BTMCs strongly expressed the HAS2 isoform on the plasma membrane (C) and (D).
Figure 3.
Cumulative signal strength in plasma membrane (A) and cytoplasm (B) was measured using Photoshop based densitometry.
Effects of TGF-β on HA production
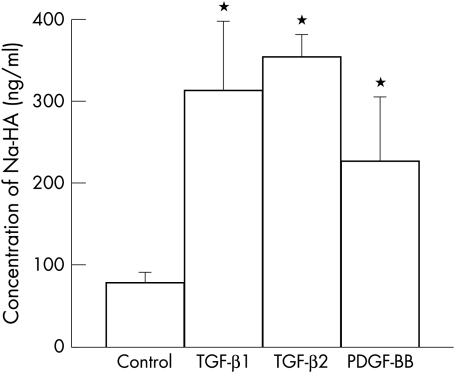
The effect of TGF-β on hyaluronan synthesis by BTMCs was observed and determined using high performance liquid chromatography (HPLC). The hyaluronan content in the medium from TGF-β treated cultures was about threefold higher and that from PDGF-BB treated cells was about twofold higher compared with that of untreated cultures (Fig 4). Thus, both TGF-β and PDGF-BB are powerful stimulators of hyaluronan synthesis in BTMCs.
Figure 4.
TGF-β and PDGF-BB stimulates hyaluronan production in BTMCs. Quiescent cells were incubated for 24 hours in DMEM medium contained 1% FBS in the absence or presence of 10 ng/ml TGF-β1, TGF-β2, or PDGF-BB. Then, hyaluronan content in media was measured by high performance liquid chromatography (n=4, error bar is SD).
DISCUSSION
In this study, we showed that BTMCs express all three HAS genes at the mRNA level, however, only HAS2 gene expression was markedly upregulated in response to TGF-β and PDGF-BB (Fig 1). The expression of HAS2 isoform in BTMCs was also confirmed at the protein level (Figs 2 and 3). The hyaluronan content in BTMCs was well correlated to the stimulatory activities of TGF-β and PDGF-BB (Fig 4). These emerging data reveal that the hyaluronan levels detected in TM are probably the result of the stimulatory activities of TGF-β and PDGF-BB. These findings are consistent with previous observations in corneal endothelium. Bovine corneal endothelial cells (BCECs) also express all three HAS isoforms, however, only HAS2 isoform was upregulated in response to TGF-β and PDGF-BB.8 It is interesting that only the HAS2, but not HAS1 nor HAS3, is upregulated by TGF-β in two different cell types of the eye—that is, BTMCs and BCECs. These results might suggest potential differences in the functions of three HAS isoforms. In addition, Pienimaki et al showed that epidermal growth factor (EGF), which promotes hyaluronan synthesis during wound healing processes, stimulated the expression of HAS2 at the mRNA level, but not that of HAS1 or HAS3 in cultured rat keratinocyte.23 These results indicate that the HAS2 isoform is responsible for hyaluronan synthesis in bovine ocular tissue and its expression is increased in cases of biological dynamic states in an inducible manner.
Hyaluronan is a key molecule in the extracellular matrices and through its interactions with specific hyaluronan binding proteins—that is, CD44, versican, and RHAMM, modulates cell motility, migration, proliferation, adhesion, development, and differentiation.2,3 Hyaluronan in TM has been suggested, in part, as a modulator of aqueous outflow resistance and TM cell survival.10–14 The constitutive expression of TGF-β and TGF-β receptors in the TM tissue18,19,24 may maintain tissue architecture attached to the anterior chamber, including TM, and may keep the homeostasis of aqueous outflow in TM, and TM cell survival though the function of HAS2 isoform. Thus, the regulation of hyaluronan in TM might be the new way to regulate aqueous outflow.
In glaucomatous eyes, elevated levels of TGF-β in aqueous humour have been reported.25,26 Further TM in glaucomatous eyes exhibited accumulation of chondroitin sulfates and a depletion of hyaluronan.13 Because pathological conditions such as glaucoma may influence many biological reactions, upregulation of TGF-β and depletion of hyaluronan in glaucoma seems contradictory at first sight. Thus, in the glaucomatous eye, stimulation of TGF-β expression in aqueous humour, followed by the induction of hyaluronan synthesis, should be promoted. However, a recent study by Knepper et al revealed that aqueous humour in primary open angle glaucoma contains an increased level of soluble CD44.23 Thus, soluble CD44 might trap the function of hyaluronan by inhibiting hyaluronan-cell surface associated CD44 interactions and thereby prevent the initiation of signalling cascades. Therefore, perturbation of endogenous hyaluronan interactions may result in increase of aqueous outflow resistance. Further, since hyaluronan production is not only dependent on TGF-β activities but also on other growth factors, such as PDGF-BB, most probably, the regulation of aqueous outflow might be complex.
Thus, increased knowledge of the modulation of HAS2 gene expression in BTMCs in vitro provides an insight into the role of hyaluronan in the aqueous outflow environment.
Acknowledgments
H Yamashita has been supported by grants in aid for scientific research from the Ministry of Education, Science, Sports, Culture, and Technology of Japan, the Ministry of Health, Labor and Welfare of Japan, the Suzuken Foundation, and the Diabetes Foundation.
REFERENCES
- 1.Lodish H, Baltimore D, Berk A, et al. Multicellularity. Molecular cell biology. 3rd ed. Chapter 24. New York: WH Freeman, 1995:1136–43.
- 2.Laurent TC, Fraser JR. Hyaluronan. FASEB J 1992;6:2397–404. [PubMed] [Google Scholar]
- 3.Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J 1993;7:1233–41. [PubMed] [Google Scholar]
- 4.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthase. J Biol Chem 1997;272:13997–4000. [DOI] [PubMed] [Google Scholar]
- 5.Itano N, Sawai T, Yoshida M, et al. Three isoforms of mammalian hyaluronan synthase have distinct enzymatic properties. J Biol Chem 1999;274:25085–92. [DOI] [PubMed] [Google Scholar]
- 6.Brinck J, Heldin P. Expression of recombinant hyaluronan synthase (HAS) isoforms in CHO cells reduces cell migration and cell surface CD44. Exp Cell Res 1999;252:342–51. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama Y, Shimada A, Sayo T, et al. . Putative hyaluronan synthase mRNA are expressed in mouse skin and TGF-β upregulates their expression in cultured human skin cells. J Invest Dermatol 1998;110:116–21. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson A, Brinck J, Briskin MJ, et al. Expression of human hyaluronan synthases in response to external stimuli. Biochemical J 2000;348:29–35. [PMC free article] [PubMed] [Google Scholar]
- 9.Usui T, Amano S, Oshika T, et al. Expression regulation of hyaluronan synthase in corneal endothelial cells. Invest Ophthalmol Vis Sci 2000;41:3261–7. [PubMed] [Google Scholar]
- 10.Lerner LE, Polansky JR, Howes EL, et al. Hyaluronan in the human trabecular meshwork. Invest Ophthalmol Vis Sci 1997;38:1222–8. [PubMed] [Google Scholar]
- 11.Knepper PA, Farbman AI, Telser A. Aqueous outflow pathway glycosaminoglycans Exp Eye Res 1981;32:265–77. [DOI] [PubMed] [Google Scholar]
- 12.Acott TS, Westcott M, Passo,MS, et al. Trabecular meshwork glycosaminoglycans in human and cynomolgus monkey eye. Invest Ophthalmol Vis Sci 1985;26,1320–9. [PubMed] [Google Scholar]
- 13.Segawa K. Ultrastructural changes of the trabecular meshwork in primary open glaucoma. Jpn J Ophthalmol 1975;19,317–22. [Google Scholar]
- 14.Knepper PA, Goossens W, Hvizd M, et al. Glycosaminoglycans of the human trabecular meshwork in primary open-angle glaucoma. Invest Ophthalmol Vis Sci 1996;37:1360–7. [PubMed] [Google Scholar]
- 15.Rittig M, Flugel C, Prehm P, et al. Hyaluronan synthase immunoreactivity in the anterior segment of the primate eye.. Graefes Arch Clin Exp Ophthalmol 1993;231:313–17. [DOI] [PubMed] [Google Scholar]
- 16.Usui T, Suzuki K, Kaji Y, et al. Hyaluronan synthase expression in bovine eyes. Invest Ophthalmol Vis Sci 1999;40:563–7. [PubMed] [Google Scholar]
- 17.Heldin C-H, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 1997;390:465–71. [DOI] [PubMed] [Google Scholar]
- 18.Tripathi RC, Borisuth NSC, Kolli SP, et al. Trabecular cells express receptors that bind TGF-b1 and TGF-b2: a qualitative and quantitative characterization. Invest Ophthalmol Vis Sci 1993;34:260–3. [PubMed] [Google Scholar]
- 19.Tripathi RC, Li J, Chang WF, et al. Trabecular cells express the TGF-b2 gene and secrete the cytokine. Exp Eye Res 1994;59:723–7. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi F, Suzuki Y, Kurihara H, et al. Molecular cloning of the bovine MYOC and induction of its expression in trabecular meshwork cells. Invest Ophthalmol Vis Sci 2000;41:2070–5. [PubMed] [Google Scholar]
- 21.Miyauchi S, Morita M, Kuramoto K, et al. Hyaluronan and chondroitin sulfate in rabbit tears. Curr Eye Res 1996;15:131–5. [DOI] [PubMed] [Google Scholar]
- 22.Pienimaki JP, Rilla K, Fulop C, et al. Epidermal growth factor activates hyaluronan synthase 2 (HAS2) in epidermal keratinocytes and increae pericellular and intracellular hyaluronan. J Biol Chem 2001;276:20428–35. [DOI] [PubMed] [Google Scholar]
- 23.Knepper PA, Mayanil CSK, Goossens W, et al. Aqueous humor in primary open angle glaucoma contains an increased level of CD44S. Invest Ophthalmol Vis Sci 2002;43:133–9. [PubMed] [Google Scholar]
- 24.Wordinger RJ, Clark AF, Agarwal R, et al. Cultured human trabecular meshwork cells express functional growth factor receptors. Invest Ophthalmol Vis Sci 1998;39:1575–89. [PubMed] [Google Scholar]
- 25.Tripathi RC, Can WF, Li J, et al. Aqueous humor in glaucomatous eyes contains an increased level of TGF-b2. Exp Eye Res 1994;59:723–7. [DOI] [PubMed] [Google Scholar]
- 26.Inatani M, Tanihara H, Katsura H, et al. Transforming growth factor-b2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol 2001;239:109–13. [DOI] [PubMed] [Google Scholar]