Abstract
Aim: To examine the relation between the type of choroidal neovascularisation (CNV) in the first eye and age related maculopathy (ARM) severity in the fellow eye.
Methods: Colour fundus photographs and fluorescein angiograms from 67 subjects with a clinical diagnosis of CNV in one eye were scrutinised. CNV was classified as wholly classic, predominantly classic, minimally classic, or occult based on the proportion of classic leakage within the lesion. ARM changes in the fellow eye were assigned a severity stage using the system described by the Rotterdam Eye Study. Logistic regression analysis was employed to examine the association between CNV subtype and ARM stage.
Results: Of subjects with classic or predominantly classic CNV in the first eye 78% exhibited least no or early ARM features in the fellow eye. By contrast, 85% of subjects with minimally classic or occult CNV in the first eye exhibited more advanced ARM features in the fellow eye. Kruskall-Wallis one way ANOVA by ranks showed that this was highly significant (p = 0.002). Logistic regression analysis showed that as the proportion of occult CNV increased in the first eye, fellow eyes of subjects in this category were more likely to have been assigned to a higher ARM stage (p = 0.019). The area occupied by the CNV in the first eye also influenced severity of ARM changes in the fellow eye.
Conclusion: The type and extent of CNV in the first affected eye has a distinct relation to ARM severity in the fellow eye. Fellow eyes of subjects with minimally classic or occult CNV in the first affected eye show widespread ARM changes suggestive of retinal pigment epithelial dysfunction. These findings suggest that classic CNV may be focal disease while occult CNV is essentially a more widespread retinal pigment epithelial disorder.
Keywords: choroidal neovascularisation, age related maculopathy
Age related macular disease is the leading cause of visual loss in developed countries and its prevalence is likely to rise as a consequence of increasing longevity.1 In early disease without vision loss, the clinical appearance of the macular retina is that of drusen, pigmentary abnormalities and atrophy, which occur in varying combinations of extent and severity. With more advancing disease, vision loss often supervenes and this may be the result of (a) extensive and confluent atrophy of the retinal pigment epithelium (RPE) and choriocapillaris resulting in a characteristic appearance termed geographic atrophy (GA), and/or (b) choroidal neovascularisation (CNV) which invades the subpigment epithelial and subretinal spaces leading to typical exudative manifestations. The international classification system has proposed a nomenclature with several subclasses in which the clinical picture is termed age related maculopathy (ARM).2 In the absence of GA or CNV, the clinical picture of varying grades of drusen and pigmentary abnormality in the central macula may be categorised into three exclusive stages of ARM (stages 1–3).3 When GA or CNV is apparent this is referred to as stage 4 ARM and is also known as dry or wet age related macular degeneration (AMD), respectively.2
An important issue for the patient and clinician is the continuing state of health of the fellow eye. AMD is a bilateral condition with a high degree of symmetrical involvement, and carries a significant risk of CNV development in the second eye once the first eye has exudative disease.4 The reported incidence of second eye involvement subsequent to initial presentation with exudative disease in one eye ranges from 4% to 12% per year.4–8 The Macular Photocoagulation Study (MPS) and other natural history studies have helped identify some of the risk factors associated with development of CNV in the fellow eye and these include greater numbers of and area covered by drusen, confluent drusen, and focal hyperpigmentation in the macular retina.4–8
It is generally accepted that for a diagnosis of CNV due to AMD to be made, the fellow eye should exhibit features of ARM. In fact, an important inclusion criterion for enrolment into the MPS trials in this patient group is the presence of ARM features in the fellow eye.7–9 A previous study10 has suggested that the characteristics of drusen are important determinants of the type of neovascular lesion. We have noticed that a significant proportion of subjects with a clinical diagnosis of classic CNV due to AMD have little or no evidence of ARM in the fellow eye. Our clinical impression was that the RPE in these fellow eyes was comparatively healthy and that the area of CNV in the first affected eye was often limited to a region that was less than one to two optic disc areas. We therefore hypothesised that the severity of ARM features in the macular retina of the second eye was related to both the type and extent of CNV in the first. The present study therefore examined the relation between the morphological features of the CNV in the first affected eye and the severity of ARM changes in the fellow eye.
PATIENTS AND METHODS
The patients included in this report were attendees at a specialist macular clinic between 1997 and 1999. Subjects attending this clinic routinely undergo visual function assessments, biomicroscopic examination of the fundus, and bilateral stereo pair colour photography. Fluorescein angiography is also performed if clinically indicated. General examination findings and clinical history are also recorded for each patient. The latter includes information on cardiovascular status (history of treated or untreated hypertension, angina, myocardial infarction, or peripheral vascular insufficiency), diabetes, any other serious disorder, medications, and smoking habit including pack years of cigarettes smoked. A standard photographic protocol for fundus photography has been in use and consists of 35° colour stereo pair photography of each macula recorded on 35 mm transparencies using the Topcon TRC 50 IX fundus camera. Digital fluorescein angiography is undertaken if clinically indicated with early frames obtained on the eye presenting with CNV and late frames from both eyes.
The photographic database was searched for cases of unilateral neovascular AMD. Inclusion criteria were a full complement of stereo angiographic frames on the eye with CNV, late angiographic frames of the fellow eye and gradable stereo pair colour fundus photographs of the fellow eye. Mounted 35 mm stereo pair colour slides of the fellow eye were sent to the grading centre of the Rotterdam Eye Study Centre.
Each pair of slides from fellow eyes was graded by two graders independently and any discrepancies were resolved by the adjudicator, an experienced ophthalmologist. The graders were unaware of the aims of the study and did not have access to colour slides or angiographic frames of the eye with the CNV. Following grading, the eye was then assigned a severity stage based on the features (Table 1) that were present within the central 6000 μm zone (radius 3000 μm) only, as described in a previous report.3
Table 1.
Staging of incident age related maculopathy (ARM): features based on the Rotterdam Study
| 0 | No signs of ARM at all or hard drusen (<63 μm) only |
| 1a | Soft distinct drusen (≥63 μm) only |
| 1b | Pigmentary irregularities only, no soft drusen (≥63 μm) |
| 2a | Soft indistinct drusen (≥125 μm) or reticular drusen only |
| 2b | Soft distinct drusen with pigmentary irregularities |
| 3 | Soft indistinct or reticular drusen with pigmentary irregularities |
| 4 | Atrophic or neovascular macular degeneration (AMD) |
Definitions of mutually exclusive stages of ARM: stage 0 represents a fundus with no drusen or pigmentary irregularities. Early ARM is subdivided into stages 1a, 1b, 2a, 2b, and 3. The case is assigned to stage 4 when there is evidence of CNV or GA.
Grading of the digital fluorescein angiograms was undertaken by two independent graders in the Belfast Angiogram Reading Centre. CNV was classified by the type of fluorescein leakage based on guidelines from the MPS and other studies.11,12 MPS guidelines define classic CNV as early distinct hyperfluorescence with well demarcated boundaries, which is seen during the early transit phase of the angiogram with leakage in the late phase that obscures the boundaries. Occult CNV is defined as hyperfluorescence which is not well delineated on angiography and the two types of occult CNV which are recognised as distinct entities are fibrovascular RPE detachments and late phase leakage of undetermined source.
The total area of the lesion was defined as the area covered by the CNV (classic or occult) plus blocked fluorescence. This blocked fluorescence usually corresponded to blood or when blood was not detected on the colour photographs presumably to some other component such as fibrous tissue and pigment. As fibrous tissue can be hyperfluorescent, when this feature was present and contiguous to the margins of the CNV, this was included in the measurements of the total area of the lesion. The total area of the lesion was measured on one of a suitable stereo pair of angiographic frames using standard templates to overlay the area of interest displayed on the computer screen. The independent contributions from each parameter—that is, classic or occult CNV, blocked fluorescence, haemorrhage and fibrosis were also ascertained by sequentially overlaying specific lesion features with templates of circles of increasing area (these circles were 1.0, 2.0, 3.0, 3.5, 4.0, 6.0, 9.0, 12.0, and 16.0 optic disc areas). Classic leakage was measured using angiographic frames taken before 30 seconds had elapsed after injection of the dye and occult leakage was measured on frames taken between 2 and 10 minutes after injection. Where fluorescein leakage accounted for all of the lesion these eyes were classified as 100% classic or occult depending upon the characteristics of the leakage.
Statistical analysis
Data were analysed using the statistical package for social sciences (spss version 11). A Kruskall-Wallis one way ANOVA was used to explore the relation between severity of ARM stage in the fellow eye and the subtypes of CNV in the first eye. For the purposes of analysis we assumed that each increasing stage of ARM represented a one point rise in severity and we assigned severity scores on a scale of 0 to 5 which were equivalent to ARM stages 0, 1A, 1B, 2A, 2B, and 3 respectively.
Also, for the purposes of analysis we subdivided the CNV into four ordered categories—occult (100% of the lesion was comprised of occult only leakage), minimally classic (1–49% of lesion is classic), predominantly classic (50–99% of the lesion is classic), and classic with no occult (100% of lesion is classic). Several studies have suggested that classic (wholly or predominantly) behave differently from occult (minimally classic or occult only) CNV.11–13 We therefore subdivided the cohort into group 1, consisting of patients with classic or predominantly classic CNV, and group 2, consisting of those with minimally classic or occult. A further analysis was then carried out using a logistic regression model to identify the relation between ARM severity in the fellow eye and the dichotomised variable (classic or occult). Other independent variables that were entered were total area of lesion, age of subject, sex, cardiovascular status, and number of pack years smoked.
RESULTS
Of the 237 cases scrutinised in the database, 67 fulfilled the inclusion criteria. The mean age of the 24 men and 43 women who comprised the group was 74 years (minimum 56 and maximum 91). Analysis of the angiograms from the 67 eyes with neovascular AMD showed that 43 were classic or predominantly classic and 24 were minimally classic or occult only lesions. Severity of ARM changes in fellow eyes ranged from 0 (having no drusen or small hard drusen only) to stage 3 ARM (Table 1).
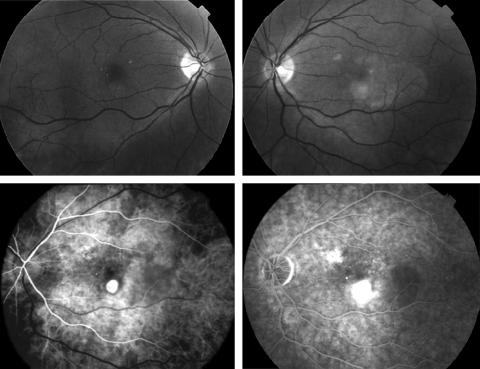
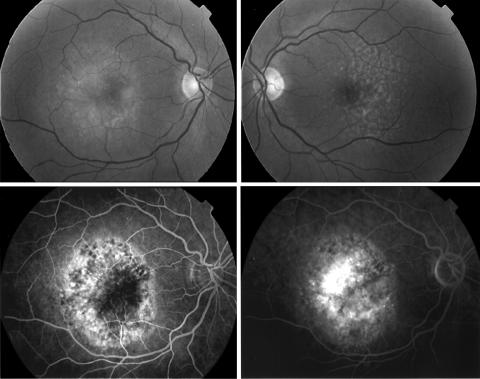
In 41 subjects with wholly or predominantly classic CNV in the first affected eye, 33 fellow eyes were classified as stage 0, 1A, or 1B ARM (80.5%), an example of which is shown in Figure 1. By contrast, 61.5% of the 26 subjects with minimally classic or occult CNV in the first eye (Table 2), had ARM features at a level of severity that was stage 2A or worse (Fig 2) . For each CNV category ranging from “classic only” to “occult only” the stage of ARM worsened progressively in severity (Table 3). Fellow eyes of patients with classic CNV in the first eye had a mean ARM severity score of 2.3 when compared with a mean score of 4.8 in fellow eyes of subjects with “occult” CNV in their first eye.
Figure 1.
Upper panels show fundus photographs of the macular retina of the right (stage 1A ) and left (stage 4) eye. Early and late phase fluorescein angiographic frames of the left eye confirm presence of a classic CNV with no occult.
Table 2.
Subdivision of CNV subtype in first eyes by ARM stage in fellow eyes. Fellow eyes of patients with classic or predominantly classic CNV in their first eye mainly exhibited ARM features at the lower end of the severity scale.
| Number of eyes | |||||
| ARM stage in fellow eyes | Classic | Predominantly classic | Minimally classic | Occult | Total |
| 0 | 10 | 4 | 3 | 0 | 17 |
| 1a | 6 | 6 | 2 | 2 | 16 |
| 1b | 3 | 4 | 1 | 0 | 8 |
| 2a | 3 | 3 | 2 | 2 | 10 |
| 2b | 0 | 0 | 0 | 0 | 0 |
| 3 | 1 | 3 | 7 | 5 | 16 |
| Total | 23 | 20 | 15 | 9 | 67 |
Figure 2.
Upper panels show fundus photographs of the macular retina of the right (stage 4) and left eye (stage 3). Early and late phase fluorescein angiographic frames of the right eye are shown in the lower panels. Fluorescein leakage typical of occult CNV is seen.
Table 3.
Mean ARM severity score in fellow eyes when categorised by CNV subtype in first eye
| CNV type in first eye (number of eyes) | Mean ARM severity score (SE) |
| Classic (23) | 2.3 (0.35) |
| Predominantly classic (20) | 3.0 (0.39) |
| Minimally classic (15) | 4.0 (0.60) |
| Occult (9) | 4.8 (0.55) |
The Kruskall-Wallis one way ANOVA by ranks demonstrated a monotonic relation between severity/extent of ARM in the fellow eye and CNV subtype in the first eye (Table 4) with a χ2 value of 14.39 on 3 degrees of freedom (p = 0.002). When the status of CNV subtype in the first eye was dichotomised into classic (classic leakage occupies 50% or more of the lesion) or occult (classic leakage occupies 49% or less of the lesion), logistic regression (Table 5) showed that subjects in this category were significantly more likely to have been assigned to a higher ARM severity stage in the second eye (p = 0.019). The total area of the lesion in the first eye also contributed to the model but just failed to reach significance (p = 0.057). Age, sex, cardiovascular disorder, and smoking status were not significant predictors for ARM severity in this model.
Table 4.
Kruskall-Wallis one way ANOVA by ranks of ARM severity scores when subjects were categorised by CNV subtype in first eye
| CNV subtype in first eye | No | Mean rank |
| Wholly classic (classic 100%) | 23 | 24.11 |
| Predominantly classic (classic 50 to 99%) | 20 | 32.85 |
| Minimally classic (classic 1 to 49%) | 15 | 41.67 |
| Occult (classic 0%) | 9 | 49.06 |
χ2 14.39, degrees of freedom 3, p = 0.002.
Table 5.
Logistic regression model with CNV subtype as the dependent variable. Independent variables were ARM severity in fellow eye, total area of CNV in the first eye, clinical and demographic parameters. There were no subjects with ARM stage 2b in this study cohort
| ARM severity scale | Exp B | p Value |
| Stage 0 | 0.06 | |
| Stage 1a | 1.43 | 0.69 |
| Stage 1b | 0.51 | 0.61 |
| Stage 2a | 3.74 | 0.16 |
| Stage 3 | 9.48 | 0.19 |
| Total area of lesion | 1.20 | 0.57 |
DISCUSSION
A previous study of 150 patients by Pauliekhoff et al showed that fellow eyes of subjects with serous pigment epithelial detachments had larger, more densely packed, and less fluorescent drusen suggesting that the characteristics of the drusen were important predictors for wet AMD.10 At the time of publication of that report, the subclassification of CNV types into the currently accepted categories had not yet been developed. More recently, the nomenclature has evolved for the classification of CNV subtypes,11,12 and methods have been developed to assess severity and extent of ARM features.2,3
In the Rotterdam Study, incidence and progression rates of ARM in the more severely affected eye were determined by stratification of ARM features to one of four exclusive stages at baseline and at follow up.3 This staging system was based on previous findings14–17 and assumed that more extensive macular changes would be associated with a higher risk of development of AMD with each successive stage of early ARM. The Rotterdam Study found that progression of early ARM stages to a more advanced stage occurred in a distinct pattern at a stable rate providing support for their model. We therefore used this method to stage the severity of ARM in the fellow eyes of our cohort. We also classified CNV in the first eye using the definitions developed in multiple clinical trials.11,12 Using the Rotterdam severity stages and MPS definitions we have shown that a clear relation exists between increasing severity of ARM characteristics in the fellow eye with increasing proportion of occult disease in the first eye. Conversely, the fellow eyes of subjects with predominantly or wholly classic CNV in the first eye tended exhibited less severe stages of ARM.
Pauleikhoff et al10 subdivided their study cohort into five groups—namely, (1) serous pigment epithelial detachments, (2) neovascular RPE detachments, (3) well defined subretinal vascular complexes, (4) early ill defined hyperfluorescence, and (5) late ill defined hyperfluorescence. Group 3 of Pauleikhoff’s study corresponds to the classic CNV subgroup in the present study. It was therefore noteworthy that Pauleikhoff et al found significantly larger and more densely packed drusen in their group 2 eyes in comparison with their group 3 eyes. Thus, the findings of the present study not only support those of Pauleikhoff et al but also suggest that occult CNV is associated with widespread RPE dysfunction whereas classic CNV may be the consequence of a more focal breakdown of the barrier between the retina and the choroid. As many pathogenetic studies implicate drusen, pigmentation, and RPE atrophy in RPE dysfunction16–23 our findings are consistent with the body of evidence that supports the view that drusen develop as a consequence of overloading and failure of the RPE over a long period of time.
Our findings also imply that classic and occult CNV occur as a result of distinct pathological processes, albeit sometimes in the same eye at adjacent locations and concur with the observations of several investigators10,24 that changes in Bruch’s membrane that induce exudative lesions should not be regarded as a single process.
Our hypothesis is also consistent with clinical findings that have been observed in many studies for which sound scientific explanations have not been forthcoming. For example classic and occult CNV respond differently to treatment by argon laser, with the former being easily ablated in its entirety, whereas treatment of the latter by argon laser simply results in the expansion of the lesion.13,22,23 More recently, clinical trials have shown a strong beneficial effect of verteporfin PDT in eyes with wholly classic or predominantly classic CNV with no effect on eyes with minimally classic CNV (TAP and VIP trials).12,25 Finally, the largest cohort study of eyes without CNV at baseline (study of fellow eyes enrolled into the MPS juxtafoveal and subfoveal trials) has shown that even those free of large drusen and hyperpigmentation have a 10% chance of developing CNV over a 5 year period indicating that the risk is not negligible.15,26
The present study which was cross sectional was not designed to answer the question whether the morphological composition of the CNV in the first eye influenced the rate of development of CNV in fellow eyes. The MPS study on the 5 year follow up of fellow eyes without neovascular AMD at baseline did not find a difference in risk of neovascularisation when subjects were characterised by the type of CNV in the first eye.22 None the less, as the present study has demonstrated a dose relation between the proportion of occult CNV in the first eye and the severity of ARM in the fellow eye there would appear to be a strong scientific rationale for examining the relation between the extent of occult CNV and risk of neovascularisation in the fellow eye.
Although we found no association between the severity of ARM in the fellow eye with smoking status, sex, or history of cardiovascular problems, the present study had limited numbers and hence limited power to identify contributions from systemic factors.
It is also limited by its cross sectional approach and its retrospective nature. None the less, our findings suggest that the health of the RPE is compromised in the fellow eyes of patients with occult CNV in the first eye and may be an important pathogenetic factor. Our data suggest that multiple pathogenetic mechanisms operating in tandem determine whether an eye will develop classic or occult CNV and which subtype will predominate. This hypothesis could be tested by longitudinal studies of fellow eyes of subjects with unilateral CNV utilising the newer technologies that can image the RPE choroidal interface with precision. The results of the present study provide clues for improved prognostic indicators and may help in designing protocols to evaluate the risk of developing CNV in the fellow eye.
Acknowledgments
The authors are grateful to Ms M Foster who provided photographic assistance and the graders Ada Hooghart and Corina Brussee working with the Rotterdam Eye Study Group. This study was in part support by a strategic project grant from the Medical Research Council, UK, Macular Disease Society of the UK, and the Optimix Foundation in the Netherlands.
REFERENCES
- 1.Evans J, Wormald RP. Is the incidence of registrable age-related macular degeneration increasing? Br J Ophthalmol 1996;80:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The International ARM Epidemiological Study Group. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Ophthalmology 1995;39:367–74 [DOI] [PubMed] [Google Scholar]
- 3.Klaver CCW, Assink JJM, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy:The Rotterdam Study. Invest Ophthalmol 2001;72:2237–41 [PubMed] [Google Scholar]
- 4.Gregor Z, Bird AC, Chisholm IH. Senile disciform macular degeneration in the second eye. Br J Ophthalmol 1977;61:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy M, Kaiser Kupfer M. Second eye involvement in age-related macular degeneration. A four year prospective study. Eye 1990;4:813–18. [DOI] [PubMed] [Google Scholar]
- 6.Pieramici DJ, Bressler SB. Age-related macular degeneration and risk factors for the development of choroidal neovascularisation in the fellow eye. Curr Opin Ophthalmol 1998;9:38–46. [DOI] [PubMed] [Google Scholar]
- 7.Macular Photocoagulation Study Group. A five year follow up of fellow eyes of patients with age-related macular degeneration. Arch Ophthalmol 1993;111:1189–99. [DOI] [PubMed] [Google Scholar]
- 8.Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol 1977;115:741–7. [DOI] [PubMed] [Google Scholar]
- 9.Macular Photocoagulation Study Group. Argon laser photocoagulation for senile macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol 1982;109:912–18. [DOI] [PubMed] [Google Scholar]
- 10.Pauleikhoff D, Barondes MJ, Minassian D, et al. Drusen as risk factors in age-related macular disease. Am J Ophthalmol 1990;109:38–43. [DOI] [PubMed] [Google Scholar]
- 11.Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the Macular Photocoagulation Study. Arch Ophthalmol 1991;109:1242–57. [PubMed] [Google Scholar]
- 12.Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Verteporfin (visudyne) therapy of subfoveal choroidal neovascularization in age-related macular degeneration. One year results of two randomised clinical trials. TAP report 1. Arch Ophthalmol 1999;117:1329–45. [PubMed] [Google Scholar]
- 13.Macular Photocoagulation Study Group. Occult choroidal neovascularization. Influence on visual outcome in patients with age-related macular degeneration. Arch Ophthalmol 1996;114:400–12. [PubMed] [Google Scholar]
- 14.Bressler NM, Muños B, Maguire MG, et al. Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities:Waterman Study. Arch Ophthalmol 1995;113:301–8. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Klein BEK, Jensen SC, et al. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1977;104:7–21. [DOI] [PubMed] [Google Scholar]
- 16.Bressler SB, Maguire MG, Bressler NB, et al. Relationship of drusen and abnormalities of retinal pigment epithelium to the prognosis of neovascular macular degeneration. Arch Ophthalmol 1990;108:1442–7. [DOI] [PubMed] [Google Scholar]
- 17.Holz FG, Wolfensberger TJ, Piguet B, et al. Bilateral macular drusen in age-related macular degeneration: prognosis and risk factors. Ophthalmology 1994;101:1522–8. [DOI] [PubMed] [Google Scholar]
- 18.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course and laser photocoagulation-induced regression. Surv Ophthalmol 1999;44:1–29. [DOI] [PubMed] [Google Scholar]
- 19.Sarks SH. Drusen and their relationship to senile macular degeneration. Aust J Ophthalmol 1980;8:117–30. [DOI] [PubMed] [Google Scholar]
- 20.Bressler NM, Bressler SB, Seddon JM, et al. Drusen characteristics in patients with exudative versus non-exudative age-related macular degeneration. Retina 1988;8:109–14. [DOI] [PubMed] [Google Scholar]
- 21.Holz FG, Wolfensberger TJ, Piguet B, et al. Bilateral macular drusen in age-related macular degeneration. Prognosis and risk factors. Ophthalmology 1994;101:1522–8. [DOI] [PubMed] [Google Scholar]
- 22.Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol 1997;115:741–7. [DOI] [PubMed] [Google Scholar]
- 23.Macular Photocoagulation Study Group. Laser photocoagulation juxtafoveal choroidal neovascularization: five-year results from randomized clinical trials. Arch Ophthalmol 1994;112:500–50 [PubMed] [Google Scholar]
- 24.Schaft TL, van der Mooy CM, Bruijn WC, et al. Histologic features of the early stages of age-related macular degeneration:a statistical analysis. Ophthalmology 1992;99:278–86 [DOI] [PubMed] [Google Scholar]
- 25.Verteporfin in PDT Report 2. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization. Am J Ophthalmol 2001;131:541–60. [DOI] [PubMed] [Google Scholar]
- 26.Macular Photocoagulation Study Group. Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Arch Ophthalmol 1993;111:1189–99. [DOI] [PubMed] [Google Scholar]