Adrenocortical carcinoma is a rare tumour with a poor prognosis. Mitotane (o,p'-DDD), a chemotherapy drug that suppresses the adrenal cortex and modifies peripheral steroid metabolism has been reported to cause ocular side effects including visual blurring, diplopia, cataract, toxic retinopathy with retinal haemorrhage, oedema, and papilloedema. We present a 32 year old woman with reduced visual acuity, retinal pigmentation, macular oedema, and abnormal ERG after taking mitotane. While primary hypoadrenalism in Addison's disease has never been reported to cause any retinal problem, secondary hypoadrenalism in adrenoleucodystrophy is associated with pigmentary retinopathy and other ocular findings. We postulate that the retinal problems secondary to mitotane treatment may act via a similar mechanism.
Case report
A 30 year old woman had a left nephrectomy, adrenalectomy, and chemotherapy in September 1997 following diagnosis of an adrenal carcinoma. In 1999, she was found to have secondary tumours in her lungs and liver. She was commenced on intra-arterial cisplatin and oral mitotane of up to 4.5 g daily for 6–8 months, ceasing in December 1999 because of weight loss, malaise and, soon after that, marked decrease of visual acuity in both eyes. The patient had no family history of any retinal disease. She had worn glasses for myopia for 9 years with best corrected visual acuity of 6/4 each eye previously.
On 2 March 2000 her visual acuity was 6/12 in the right eye and 6/60 in the left eye. She also had facial pigmentation. Funduscopy showed extensive pigmentary clumping in each eye and macular oedema in the left side (Fig 1). She was commenced on cortisone acetate and fludrocortisone to attempt to improve her vision.
Figure 1.
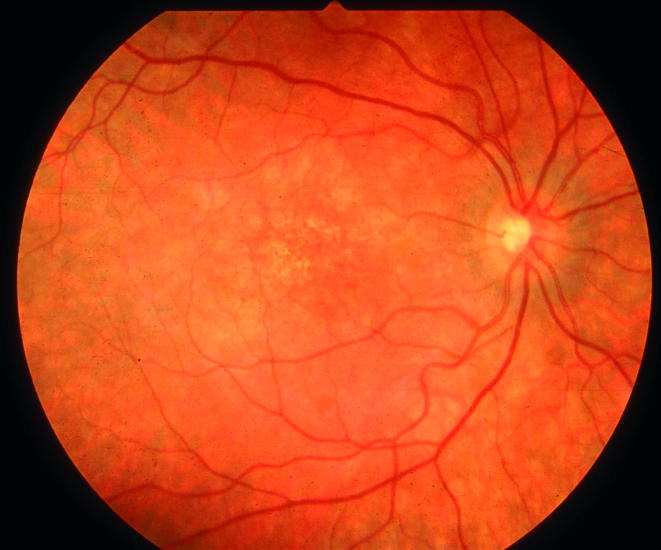
Right fundus showing mottled pigmentation at the macula.
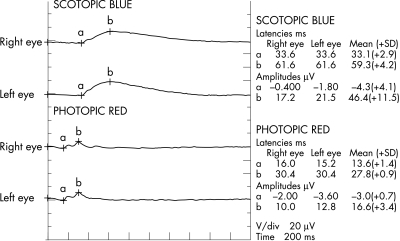
One week later her visual acuity was 6/18 in the right and 6/24 in the left. Fundus examination showed mottled pigmentation at the macula and mid-periphery of both eyes but macular oedema in her left eye had resolved. Disc and vessels were normal. Full field electroretinography (ERG) was performed. Her rod response (scotopic, dim blue stimulus) b-wave was 50% of normal, the cone response (photopic, red stimulus) had b-wave 75% of normal amplitude and delayed implicit time (Fig 2). The amplitude of 30 Hz flicker responses was also reduced. There was no significant asymmetry between the two eyes. On 30 March 2000, her visual acuity improved to 6/12 in the right and 6/9 in the left. Her facial pigmentation had also faded.
Figure 2.
Full field ERG.
Comment
Surgical resection is the treatment of choice for adrenocortical carcinoma.1 Mitotane (o,p'-DDD) is the only drug that causes regression of metastases and improves survival.2,3 Its biochemical action is unknown but data suggest that it modifies peripheral steroid metabolism and directly suppresses the adrenal cortex. The incidence of ocular side effects was 4% in a study of 132 patients.3 These effects include visual blurring, diplopia, lens opacities, optic neuritis, and a toxic retinopathy with features of papilloedema and retinal haemorrhage. In another study involving 19 patients, three patients had toxic retinopathy that included papilloedema, small retinal haemorrhages, and oedema and another patient had a subcapsular cataract.4 To our knowledge neither pigmentary retinopathy nor abnormal ERG findings have been reported previously. Previous studies also did not mention side effects reversibility except for a case of lenticular opacities that disappeared 5 days after discontinuance of mitotane.3 In our case, the patient's visual acuity did improve significantly, with drying out of macular oedema after cessation of therapy and initiation of steroid replacement.
It is possible, however, that the ocular changes in this patient were not caused by mitotane but were secondary to cancer associated retinopathy (CAR). Its characteristic findings include attenuated retinal arterioles, with limited, if any, clinically apparent retinal pigmentary changes and cells in the vitreous humour.5 CAR was considered to be unlikely in this case because of the normal calibre of the retinal arterioles, the absence of vitreous cells, and the timing of the onset. Patients with CAR experience visual symptoms that often precede or are concurrent with the tumour diagnosis.6
Although primary hypoadrenalism in Addison's disease has never been reported to cause retinal problems, secondary hypoadrenalism in adrenoleucodystrophy is associated with pigmentary retinopathy and other ocular findings. Adrenoleucodystrophy is a group of rare lipid storage disorders with increased serum level of long chain fatty acids (C24–C30). Ocular findings in adrenoleucodystrophy include visual loss secondary to visual tract demyelination and primary retinal ganglion cell degeneration, squint, cataracts, loss of corneal sensation, abnormal visual evoked potentials, and macular pigmentary changes.7 The retinal pigmentary changes observed histologically were different from those in retinitis pigmentosa.8
We postulate that the retinal side effects of mitotane could occur via a similar mechanism that affects the metabolism of long chain fatty acids. Mitotane causes a reduction in plasma 17-hydroxy corticosteroids level but an increase in the levels of 6-beta-hydroxyl cortisol, cholesterol, liver enzymes, corticosteroid binding globulin, and sex hormone binding globulin.1 At the time of writing, there was no published information on whether mitotane treatment affects serum long chain fatty acid levels. The availability of such data will be valuable for evaluating this postulation.
References
- 1.Luton J-P, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors and the effect of mitotane therapy. N Engl J Med 1990;322:1195–201. [DOI] [PubMed] [Google Scholar]
- 2.Lubitz JA, Freeman L, Okun R. Mitotane use in inoperable adrenal cortical carcinoma. JAMA 1973;223:1109–12. [PubMed] [Google Scholar]
- 3.Hutter AM, Kayhoe DE. Adrenal cortical carcinoma, results of treatment with o,p'-DDD in 138 patients. Am J Med 1966;41:581–92. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman DL, Mattox VR. Treatment of adrenocortical carcinoma with o,p'-DDD. Med Clin North Am 1972;56:999–1012. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Ann Neurol 1990;28:162–67 [DOI] [PubMed] [Google Scholar]
- 6.Fishman GA, Birch DG, Holder GE, et al. Electrophysiologic testing in disorders of the retina, optic nerve and visual pathway. 2nd ed. San Francisco: The Foundation of the American Academy of Ophthalmology, 2001:118–120.
- 7.Traboulsi EI, Maumenee IH. Ophthalmologic manifestations of X-linked childhood adrenoleukodystrophy. Ophthalmology 1987;94:47–52. [DOI] [PubMed] [Google Scholar]
- 8.Glasgow BJ, Brown HH, Hannah JB, et al. Ocular pathologic findings in neonatal adrenoleukodystrophy. Ophthalmology 1987;94:1054–60. [DOI] [PubMed] [Google Scholar]