Abstract
Aim: To study the ocular bioavailability of a triple dose, single application of sodium fluorescein to the human anterior segment from a novel drug delivery device.
Methods: In a randomised, open label study 22 healthy volunteers applied a single lyophilisate to one eye (+1 minute) and three conventional eye drops (+1, 16, 31 minutes) of fluorescein ophthalmic solution to the fellow eye. The fluorescein dose of the lyophilisate was 204 mg corresponding to three conventional, preservative-free eye drops of 40 ml fluorescein SE Thilo 0.17% (68 μg each) (Alcon). Fluorophotometry was performed (Fluorotron Master II Ocumetrics, USA) before and +15, 30, 45, 60, 120, 180, 240, 300, 360, 420 minutes after application. The fluorescein concentrations of the corneal stroma and mid-anterior chamber were analysed by paired t test.
Results: Cornea and anterior chamber mean values (ng/ml) were significantly higher (p<0.018, paired t test) in the lyophilisate group up to 7 hours after application with the exception of +45 minutes. The mean fluorescein bioavailability from the lyophilisate was up to 11 times higher in the cornea and up to 8.7 times higher in the anterior chamber compared with the three preservative-free eye drops.
Conclusion: A triple dose was delivered to the human eye with a single lyophilisate application for the first time. A significantly better bioavailability was achieved in the cornea and anterior chamber for up to 7 hours by means of drug application with lyophilisates. The application of medications by means of the lyophilisate will improve the treatment of, for example, glaucoma, bacterial, viral and fungal infections, as well as dry eye syndrome.
Keywords: bioavailability, drug delivery, fluorophotometry, glaucoma
In the past there have been numerous attempts to create new application forms like nanoparticles, mucoadhesive polymers, ocular inserts, corneal collagen shields, and soluble ophthalmic delivery systems. Many drug delivery devices such as the collagen shield, NODS or Ocusert have been demonstrated to be safe and tolerated by the eye.1–4 However, none have been used therapeutically on a broad scale. Conventional dosage forms represent nearly 90% of the currently marketed formulations.5
Conventional eye drops are still a surprisingly problematic dosage form. Patients find it difficult to administer a single drop accurately. Elderly patients or children may injure the surface of their eye or cause bacterial contamination due to contact with the tip of the bottle (Fig 1). The low viscosity of the conventional eye drops results in an incalculable volume which drains out of the eye immediately after application leading to uncontrollably varying bioavailability of medications inside the eye.
Figure 1.
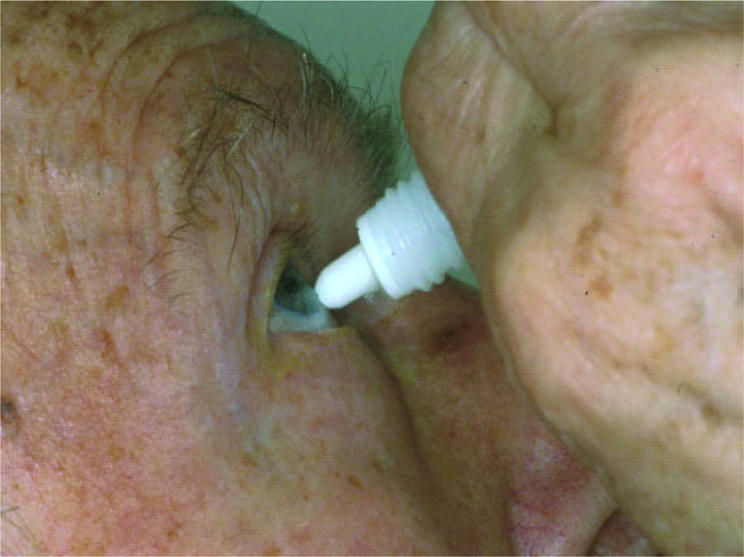
Patient contaminating the medication and injuring the eye surface.
An ideal application form should meet the following criteria: easy use; neutral pH value and absence of preservatives, constant drug inflow into the eye; sterile single dosage; minimal discomfort; minimal influence on visual acuity.5
The safety and tolerability of the lyophilisate as a new drug delivery device was studied successfully.2,6 In comparison with a conventional, preservative-free tear film substitute it demonstrated a very good tolerability and excellent safety (Fig 2).
Figure 2.

The lyophilisate.
In this study we compared the ocular bioavailability of a triple dose of fluorescein from a single lyophilisate, a novel preservative-free, freeze dried ophthalmic drug delivery system with the one of three conventional eye drops.
SUBJECTS AND METHODS
Participants
Twenty two healthy white volunteers (13 males, nine females) with a mean age of 29 (SD 5) years (minimum 22, maximum 45 years) were enrolled in the study. None of the volunteers had a previous history of eye disease, operations, diabetes mellitus, local or systemic medication, contact lens wear, myopia or hyperopia > 6 dioptres.
In a randomised, open label study a single lyophilisate was applied to one eye of each of 22 healthy volunteers and three conventional fluorescein eye drops of the same total dose to the fellow eyes. For reasons of randomisation the participants were divided into two groups. One group received one lyophilisate to the left eye and three preservative-free eye drops to the right eye, while the other received one lyophilisate to the right eye and three preservative-free eye drops to the left eye.
The fluorescein dose of the lyophilisate was 204 μg corresponding to three conventional, preservative-free eye drops of 40 μl fluorescein SE Thilo 0.17% (68 μg each) (Alcon, Germany).
In order to avoid the three conventional eye drops from immediately rinsing out of the lower eye lid as a result of their higher volume, they were applied at 15 minute intervals at +1, +16, and +31 minutes. Therefore, the total dose of 204 μg fluorescein in both eyes was first achieved at the +45 minute measurement.
Fluorophotometry of the corneal stroma (C) and mid-anterior chamber (AC) was performed with the Fluorotron Master II (Ocumetrics, Palo Alto, CA, USA) before and +15, +30, +45, +60, +120, +180, +240, +360, +420 minutes after the first application.
The fluorescein lyophilisate was deposited in the lower cul de sac by stripping it off the carrier in a wiping motion over the rim of the lower eye lid (Fig 3). Upon contact with the conjunctiva the lyophilisate rehydrates rapidly in the tear film. With every blink of the upper lid the fluorescein is spread over the cornea and conjunctiva diluted by the tear film (Fig 4).5 In contrast with conventional ophthalmic eye drops less medication is lost with blinking and tearing.
Figure 3.

Application of a lyophilisate.
Figure 4.
Fluorescein at the cornea.
To create the fluorescein lyophilisate, fluorescein was dissolved in a 1.0 % aqueous solution of hydroxypropylmethyl cellulose (HPMC, Methocel E50) filtered through a 0.22 μm mixed cellulose ester filter and deposited onto steam sterilised flexible hydrophobic poly(tetrafluoroethylene) (PTFE) carrier strips (0.1 mm thick) on a laminar flow workbench. The strips were deep frozen at −30°C for 45 minutes and freeze dried (Christ alpha 2-4, Osterode, Germany) for 2 hours at 1.2 mbar under aseptic conditions and packed aseptically in presterilised test tubes.
The tolerability of both application forms was assessed by means of 100 mm visual analogue scales. All volunteers documented the quality of discomfort 1 minute after the lyophilisate or first eye drop application (+1 minute) and 1 minute after the last conventional eye drop application (+46 minutes). The value 0 mm denoted no discomfort, whereas the value 100 mm represented highest discomfort.
Fluorescein concentrations (ng/ml) of the C and the AC were compared by paired t test (SPSS 10.0 for Windows, Statistical Package for the Social Studies, Chicago, IL, USA).
RESULTS
All volunteers completed the study protocol. One measurement could not be carried out on two participants (Nos 12, 17). Two participants (Nos 8, 19) did not apply the lyophilisate properly. The lyophilisate remained at the outer rim of the lower eyelid and did not reach the cornea. In one participant (No 4) an abnormal anatomy of the lower eyelid was recorded which may have led to the high cornea fluorescein concentrations.
The baseline measurements (autofluorescence profile) of both groups before application of eye drops or lyophilisates showed no significant difference in the AC autofluorescence (p <0.474; mean 3.6 (SD 1.5) v 3.7 (1.7) ng/ml) but a significant difference in the cornea autofluorescence (p <0.047; mean 10.8 (2.6) v 9.7 (2.8) ng/ml) which was the result of randomisation and not due to abnormal autofluorescence values (Table 1).
Table 1.
| Anterior chamber | Cornea | |||||||||
| Lyophilisate | Eye drop | Lyophilisate | Eye drop | |||||||
| Time (minutes) | Mean | SD | Mean | SD | p Values | Mean | SD | Mean | SD | p Values |
| 0 | 3.6 | 1.5 | 3.7 | 1.7 | <0.474 | 10.8 | 2.6 | 9.7 | 2.8 | <0.047 |
| 15 | 64.7 | 68.7 | 7.4 | 7.0 | <0.001 | 2193.1 | 1755.2 | 193.5 | 332.0 | <0.000 |
| 30 | 16.2 | 22.0 | 5.2 | 2.6 | <0.018 | 407.5 | 574.2 | 74.4 | 64.1 | <0.000 |
| 45 | 7.5 | 4.5 | 7.3 | 6.8 | <0.848 | 88.0 | 74.7 | 139.3 | 166.1 | <0.000 |
| 60 | 9.8 | 5.9 | 4.9 | 2.5 | <0.000 | 65.7 | 38.4 | 35.3 | 28.5 | <0.001 |
| 120 | 17.9 | 11.3 | 6.8 | 3.9 | <0.000 | 60.6 | 42.7 | 21.3 | 9.1 | <0.000 |
| 180 | 19.2 | 12.8 | 7.9 | 4.2 | <0.000 | 53.5 | 35.1 | 21.4 | 9.7 | <0.000 |
| 240 | 17.6 | 10.8 | 7.8 | 4.2 | <0.000 | 48.7 | 27.8 | 21.2 | 8.5 | <0.000 |
| 300 | 15.5 | 9.7 | 7.0 | 3.8 | <0.000 | 40.7 | 18.6 | 19.4 | 7.4 | <0.000 |
| 360 | 13.9 | 8.8 | 6.8 | 3.5 | <0.000 | 36.2 | 17.5 | 21.4 | 18.0 | <0.020 |
| 420 | 12.8 | 8.5 | 6.3 | 3.0 | <0.000 | 35.9 | 16.6 | 18.6 | 7.6 | <0.000 |
Cornea concentrations (C)
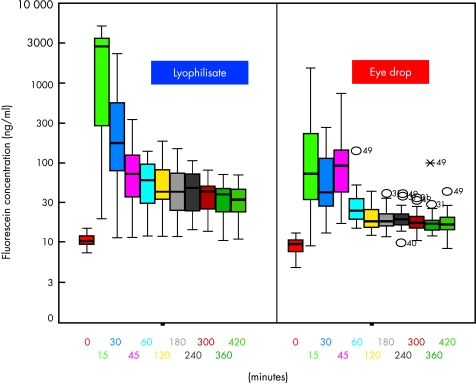
With the exception of +45 minutes all mean fluorescein concentrations of the corneal stroma were significantly higher in the lyophilisate group up to 7 hours after application (p <0.02). At +45 minutes the fluorescein concentration of the eye drop group was 1.6 times higher (mean 139.3 ng/ml v 88.0 ng/ml) than in the lyophilisate group (Table 1). After the comparison base at +45 minutes, when the same total dose was applied, the C mean fluorescein concentration of the lyophilisate treated eyes reached 65.66 ng/ml at +60 minutes and decreased to 35.89 ng/ml at +420 minutes. The C mean fluorescein concentration of the conventional eye drop treated eyes reached 35.32 ng/ml at +60 minutes and decreased to 18.57 ng/ml at +420 minutes. The mean fluorescein concentrations after lyophilisate application were 11.3 times (+15 minutes, partly due to the not yet equal applied doses; 2193.1 ng/ml v 193.5 ng/ml), over 2.8 times (+120 minutes, equal applied doses; 60.6 ng/ml v 21.3 ng/ml) to 1.7 times (+ 360 minutes; 36.2 ng/ml v 21.4 ng/ml) higher (Table 1, Fig 5).
Figure 5.
Fluorescein concentration flow over time in the cornea: lyophilisate v eye drop; Box plot diagrams: the horizontal lines in the box denote the 25th, 50th, and 75th percentile values. The error bars denote the fifth and the 95th percentile values. The square symbol in the box shows the mean of the data column.
Anterior chamber concentrations (AC)
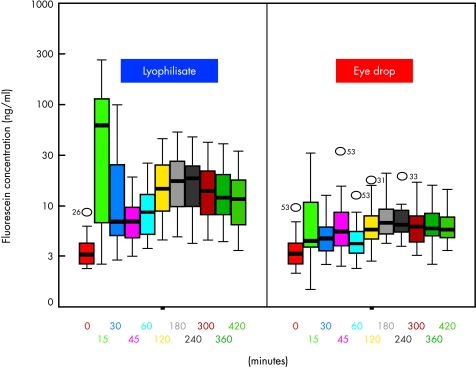
All mean fluorescein concentrations of the mid-anterior chamber were higher in the lyophilisate group. All values after +45 minutes were statistically significant (p<0.018) in favour of the lyophilisates. After the starting comparison at +45 minutes when the same total dose was applied to both eyes the fluorescein concentrations at the AC did not differ significantly (p=0.848). The AC mean fluorescein concentration of the lyophilisate treated eyes reached a maximum of 19.15 ng/ml at +180 minutes and decreased to 12.79 ng/ml at +420 minutes (Table 1). The AC mean fluorescein concentration of the conventional eye drop treated eyes reached a maximum of 7.87 ng/ml at +180 minutes and decreased to 6.30 ng/ml at +420 minutes. The mean fluorescein concentrations after lyophilisate application were 8.7× (+15 minutes, partly due to not yet equal applied doses; 64.7 ng/ml v 7.4 ng/ml), over 2.7× (+120 minutes, equal applied doses; 17.9 ng/ml v 6.8 ng/ml) to 2.0× (+420 minutes; 12.8 ng/ml v 6.3 ng/ml) higher (Table 1, Fig 6).
Figure 6.
Fluorescein concentration flow over time in the anterior chamber: lyophilisate v eye drop; Box plot diagrams: the horizontal lines in the box denote the 25th, 50th, and 75th percentile values. The error bars denote the fifth and the 95th percentile values. The square symbol in the box shows the mean of the data column.
Tolerability
The mean discomfort value for the lyophilisate application was 52 mm. The mean discomfort value for the first and last eye drop application was 19 mm each. The triple dose lyophilisate application studied showed to be less comfortable than the single dose lyophilisate used in recent studies by Diestelhorst et al and Dinslage et al.2,5,6 Since all participants received three eye drop applications at a discomfort of 19 mm each, the total discomfort value for the conventional eye drop applications was calculated as 57 mm, thus 5 mm more than the single lyophilisate application value.
DISCUSSION
The triple dose, single application of a medication applied to the human living eye by means of a lyophilisate was studied for the first time. So far no drug delivery system has been reported to be able to deliver such a high dose of a medication to the surface of the human living eye at one time.
Up to 7 hours after the application of a triple dose, single lyophilisate significantly higher values of fluorescein were found in both the corneal stroma (C) and the mid-anterior chamber (AC) (Table 1) at all time points. There was no significant difference (p <0.848) at the initial comparison basis at +45 minutes (7.5 ng/ml v 7.3 ng/ml). The mean fluorescein concentrations were up to 2.8 times higher in the C (60.6 ng/ml v 21.3 ng/ml) and up to 2.7 times higher in the AC (17.9 ng/ml v 6.8 ng/ml) after equal doses were given. At +15 minutes the lyophilisate group showed 11.3 times higher fluorescein concentrations in the C (2193.1 ng/ml v 193.5 ng/ml) and 8.7 times higher fluorescein concentrations in the AC (64.7 ng/ml v 7.4 ng/ml). The following measurements showed a significantly higher (p=0.000) mean fluorescein concentration from lyophilisate application.
Our findings concur with the findings of Dinslage et al.5 In their study the lyophilisate contained a dose of 68 μg of fluorescein corresponding to a single preservative-free, conventional eye drop of 40 μl fluorescein 0.17% (Alcon, Germany). The authors found significantly higher mean values in the C and AC from the lyophilisate in comparison with a single conventional eye drop. They reported still increasing mean fluorescein concentrations in the AC even 3 hours after lyophilisate instillation.5 Our study confirms their findings. In our study the mean fluorescein concentration in the AC after lyophilisate instillation increases to a maximum after 3 hours and then slowly decreases (Table 1, Fig 6). Since direct non-invasive objective measurements of antiglaucomatous medications or antibiotics in the living human anterior chamber were not available, anterior chamber fluorophotometry (Fluorotron Master II; Ocumetrics, Palo Alto, CA, USA) was used as a non-invasive, observer independent comparison of the two drug delivery systems. Regarding their results the drug absorption from the new application form seems to be superior to a single preservative-free, conventional eye drop.
Our data underline the possibility of delivering higher doses of medications to the human living eye by means of a single lyophilisate application. The lyophilisate is a water free, pH neutral and preservative-free drug delivery system which avoids the rapid dilution of medications at the tear film. Owing to its superior bioavailability, easy handling and good safety this device may improve the medical treatment of eyes with glaucoma, eyes with bacterial, viral or fungal infections, and in dry eye syndrome. In all cases chronic application of medications over a longer period of time is required. Using conventional eye drops most of the medication is lost immediately after application because of volume, tearing, and blinking and as a result of the foreign body sensation caused by preservatives. This was confirmed in the study of Dinslage et al.5 The authors found cornea concentrations barely exceeding autofluorescence values +15 minutes after application of 40 μl of a fluorescein 0.17% eye drop. The anterior chamber concentrations did not increase statistically significantly at all time points up to 3 hours after application.
The absorption of an ocular medication in the cul de sac commences by diluting the drug when it is mixed with the tear fluid. The efficiency of ocular drug absorption depends on the time the medication remains in the precorneal tear film,7 the time available for the diffusion of the drug into the eye. Another important route of drug loss from the precorneal area is the non-corneal absorption, which mostly occurs via the conjunctiva. Its surface area is greater in order of magnitude than the cornea and it is 2–30 times more permeable depending on the drug.8 One of the major disadvantages of the topical ophthalmic water solutions and suspensions is the rapid and extensive precorneal loss caused by drainage and high tear fluid turnover. The contact time of the drug with ocular tissues is relatively short (∼1 minute) because of the permanent production of lacrimal fluid (0.5–2.2 μl/min).9 When the typical conventional eye drop volume of 35–50 μl is added onto the precorneal tear film of approximately 7–9 μl,10 the greater part of the drug is rapidly drained from the surface via nasolacrimal duct. At least 80% of the applied medication disappears via lacrimal drainage and not by entering the eye.11,12
While a non-irritated eye has an average tear turnover rate of 16% per minute, the instillation of an eye drop stimulates lacrimation to increase the turnover rate to 30% per minute. Reflex tearing due to stinging, upon instillation of an irritating preservative or drug, produces a higher loss rate.13 Physical and emotional factors can also increase lacrimation. The volume of reflex tears varies from 3 to 400 μl. Increased blinking, due to instillation of irritating preservatives, eliminates about 2 μl per blink out through the puncta into the nasolacrimal duct.7 Because of this reflex tear production and the dilution factor the effect of a conventional topical ophthalmic eye drop is quite uncontrollable and of shorter duration. All these factors cause less than 3% of an applied dose to reach the aqueous humour, which is one reason why high concentrations are used in conventional ocular therapy.
Topical instillation of ophthalmic solutions relies on “pulse entry” of the drug which is characterised by a rapid rise followed by a rapid decline in the ocular drug concentration. Adequate topical therapy can thus either be achieved with a high level of pulse entry or via a sustained release effect. The former, in the form of conventional eye drops, is used today with disadvantages and the necessity of frequently irritating the eyes. The latter method can be achieved by means of the lyophilisate.
The rehydration of the lyophilisate commences immediately after administration to the conjunctival fold. There is no additional volume instilled onto the precorneal surface of the eye and no drug instantly draining out of the eye can be seen. The entire drug remains in the cul de sac. With higher drug concentrations in the tear film, because of the steady tear volume, a sustained release and better inflow to ocular surface tissues and aqueous humour are achieved. Consequently more exact dosing can be achieved compared to conventional eye drops.
In two patients the lyophilisate was not applied properly. This was most probably because of technical production alterations of the samples. In those two samples the rehydration had already started on the carrier so that the lyophilisate stayed attached to it and could not be stripped off properly.
In this study we did not analyse whether the difficulties of lyophilisate application increases with age. Data on file of volunteers of all different ages applying the lyophilisate indicate that this is not the case. Even elderly volunteers could apply the lyophilisate properly without practice. Of course concomitant ocular diseases may cause problems in applying the lyophilisate. This is, however, much more the case in the application of a conventional eye drops. In contrast to the lyophilisate application, the tactile sense can not be used with the eye drop bottle or the patient would not hurt his conjunctiva or cornea.
Lyophilisates containing antibiotics or antimycotics will achieve a much higher concentration in the corneal stroma in the treatment of corneal infections, when a fortified therapy is necessary. Lyophilisates containing antiglaucoma medications will be able to maintain a relatively high and steady concentration of the substance in the anterior chamber over a longer period of time. Thus, intraocular pressure (IOP) will be maintained at a lower level with lesser diurnal fluctuation. The application intervals can be longer, resulting in more comfort to the patient as well as better compliance.
CONCLUSIONS
The lyophilisate is a new application form that has superior bioavailability compared with conventional eye drops. A triple dose can be administered to the human eye with a single lyophilisate application and results in significantly higher cornea and anterior chamber concentrations for up to 7 hours after application. Our data confirm that lyophilisates are a favourable alternative to conventional eye drops. A significantly better bioavailability can be achieved by means of drug application with lyophilisates in human eyes. A well tolerated delivery device is not only important to the comfort and compliance of our patients but should also lead to higher drug concentrations inside the eye.
Acknowledgments
We would like to thank Dipl-Stat Silke Coburger, University of Cologne for her help with statistics.
M Diestelhorst, MD, and R Süverkrüp, PhD, have a proprietary interest in the device.
References
- 1.Alani SD. The ophthalmic rod: a new ophthalmic drug delivery system. Graefes Arch Clin Exp Ophthalmol 1990;228:297–301. [DOI] [PubMed] [Google Scholar]
- 2.Diestelhorst M, Krieglstein GK. The ocular tolerability of a new ophthalmic drug delivery system (NODS). Int Ophthalmol 1994;18:1–4. [DOI] [PubMed] [Google Scholar]
- 3.Shell JW. Ophthalmic drug delivery systems. Drug Dev Res 1984;6:245–61. [DOI] [PubMed] [Google Scholar]
- 4.Le Bourlais C, Acar L, Zia H, et al. Ophthalmic drug delivery systems—recent advances. Prog Ret Eye Res 1998;17:33–58. [DOI] [PubMed] [Google Scholar]
- 5.Dinslage S, Diestelhorst M, Weichselbaum A, et al. Lyophilisates for drug delivery in ophthalmology. Pharmacokinetics of fluorescein in the human anterior segment. Br J Ophthalmol (in press). [DOI] [PMC free article] [PubMed]
- 6.Diestelhorst M, Grunthal S, Süverkrüp R. Dry drops: a new preservative-free drug delivery system. Graefes Arch Clin Exp Ophthalmol 1999;237:394–8 [DOI] [PubMed] [Google Scholar]
- 7.Maurice DM, Mishima S. Ocular pharmacokinetics. In: Sears ML, ed. Pharmacology of the eye. Handbook of experimental pharmacology. New York: Springer-Verlag.1984;69:19.
- 8.Ahmed I, Gokhale RD, Shah MV, et al. Physicochemical determinants of drug diffusion across the conjunctiva, sclera and cornea. J Pharm Sci 1987;76:583–6. [DOI] [PubMed] [Google Scholar]
- 9.Sugrue MF. The pharmacology of antiglaucomatous drugs. Pharm Ther 1989;43:91–138. [DOI] [PubMed] [Google Scholar]
- 10.Mishima S, Gasset A, Klyce SD, et al. Determination of tear volume and tear flow. Invest Ophthalmol 1966;5:264–76. [PubMed] [Google Scholar]
- 11.Patton TF, Francoeur M. Ocular bioavailability and systemic loss of topically applied ophthalmic drugs. Am J Ophthalmol 1978;85:225. [DOI] [PubMed] [Google Scholar]
- 12.Chrai SS, Makoid MC, Eriksen SP, et al. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci 1974;63:333. [DOI] [PubMed] [Google Scholar]
- 13.Farris RL, Stuchell RN, Mandell ID. Basal and reflex human tear analysis. I Physical measurements: osmolarity, basal volumes, and reflex flow rate. Am Acad Ophthal 1981;88:852. [DOI] [PubMed] [Google Scholar]