Abstract
Background: Leber’s congenital amaurosis (LCA) accounts for 5% of inherited retinal disease and is usually inherited as an autosomal recessive trait. Genetic and clinical heterogeneity exist. Mutations have been described in the RPE65, CRB1, RPGRIP1, AIPL1, GUCY2D, and CRX genes and other pedigrees show linkage to the LCA3 and LCA5 loci. The latter is a new locus which maps to 6q11-q16. The ocular findings and the evolution of the macula staphyloma are described in five members of a Pakistani family with consanguinity and a mutation in the LCA5 gene.
Methods: 13 family members including five affected individuals consented to DNA analysis and ocular examination including fundal photography.
Results: Ocular abnormalities are described. The most striking feature was the progression of macula abnormalities in three brothers resulting in a colobomatous appearance in the eldest compared to only mild atrophy in the youngest. The phenotypic pattern of this mutation in this Pakistani family contrasts with the “Old Order River Brethren” who were of Swiss descent, in whom the mutation was first described.
Conclusion: The evolution of a new phenotypic picture is presented to a mutation in LCA5.
Keywords: Leber’s congenital amaurosis, DNA analysis
Leber’s congenital amaurosis (LCA) is the name ascribed to a group of inherited retinal dystrophies representing the commonest genetic cause of visual impairment in infants and children.1 The heritable nature and consanguinity as an association were recognised by Leber in the original report of this disorder.2 Subsequent reports confirmed the majority of affected pedigrees exhibit an autosomal recessive mode of inheritance.3, 4 Locus heterogeneity was originally suspected in 1963, from the observation that unaffected children were born to parents with phenotypically the same disorder.5
Currently six genes in which mutations are implicated in the pathogenesis of LCA have been cloned,6–11 and three further loci are known to exist from linkage studies.12–15 In one genetic survey assessing the frequency of mutations in three known genes considered to have a major role in LCA, only 15.9% of the sample population harboured disease-causing mutations.16 This implies a substantial proportion of cases with LCA result from mutations in as yet unidentified genes. This phenotypic variation seen in LCA is a consequence of this genetic heterogeneity.
The spectrum of clinical features seen in LCA is well documented,17 as is the natural history in this disorder.18 However, the descriptions from these large series describe trends of disease progression in groups of individuals with different genetic defects.
This report presents a unique chronological window into the phenotype and progression of LCA within a single consanguineous family, in whom genetic analysis has confirmed linkage to the LCA5 locus on 6q11-16.12
METHODS
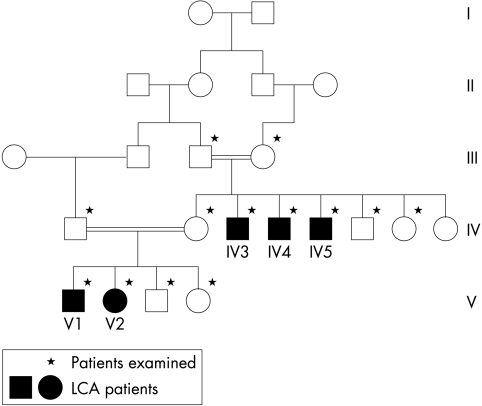
We examined 13 members of a large consanguineous pedigree, which included five affected individuals (Fig 1). The family was identified via our ocular genetics research clinic in Pakistan and all the individuals were subject to a full ophthalmic examination by two independent ophthalmologists using direct and indirect ophthalmoscopy through dilated pupils. Inclusion criteria for diagnosis with LCA included documented poor vision from early infancy and exclusion of other retinal disorders. Electrodiagnostic tests were not available to us in this rural developing world setting. However, in view of the similar symptomatology between the affected individuals, it was assumed they all expressed an identical disease pattern.
Figure 1.
Family tree of subjects examined.
Fundus photographs were taken of the eldest three brothers (Figs 2, 3, and 4) whose ages ranged between 21 and 30 years. Genomic DNA extraction was performed from peripheral blood leucocytes using standard techniques. Genotyping was performed by choosing microsatellite markers flanking the LCA5 region. Standard polymerase chain reaction (PCR) techniques were employed to generate allele fragments which underwent electrophoresis on an ABI 377 automated DNA analyser and analysed using GeneScan 3.1.2 and Genotyper 2.0 software. Using the Cyrillic pedigree drawing package and linksys data management package, the linkage suite of programs was utilised to generate a lod score.
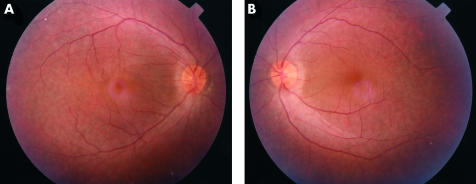
Figure 2.
Subject IV5 aged 21: minimal atrophy at macula. (A) Right fundus, (B) left fundus
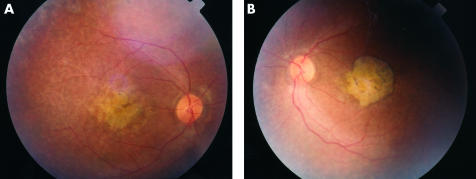
Figure 3.
Subject IV4 aged 25: atrophy at macula with pigment rim and clumps. (A) Right fundus, (B) left fundus
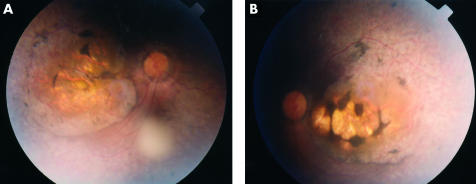
Figure 4.
Subject IV3 aged 30: macula staphyloma. (A) Right fundus, (B) left fundus
RESULTS
Linkage to the LCA5 locus was confirmed by linkage analysis. A multipoint lod score of 3.75 was generated using microsatellite markers D6S391 and D6S968 against LCA5. All affected individuals had a non-syndromic form of LCA with normal neurological examination and intelligence. Ocular examination revealed a best corrected visual acuity of perception of light. Pendular nystagmus was present in the three adults and roving eye movements in the two children (Table 1).
Table 1.
Subject characteristics
| Subject | Age | Eye movement | Vitreous | Macula | White dots | Spiculation |
| V2 | 2 | Roving | Clear | Not seen | Not seen | Not seen |
| V1 | 6 | Roving | Clear | Minimal atrophy | Equatorial | None |
| IV5 | 21 | Pendular nystagmus | Opacity LE | Atrophy | Equatorial | Equatorial |
| IV4 | 25 | Pendular nystagmus | Opacity RE | Atrophy and pigment clumps | Equatorial | None |
| IV3 | 30 | Pendular nystagmus | Opacity LE | Staphyloma | Equatorial | Peripheral and equatorial |
The anterior segment examination was essentially normal with no evidence of keratoconus or cataract. The vitreous was clear in the younger family members but a vitreous opacity was noticed unilaterally in the three elder brothers.
Fundal examination revealed bilateral and symmetrical retinal changes. Detailed fundal examination of the youngest child was not possible in view of the roving eye movements. In the other four individuals retinal vessel attenuation was noticed and “waxy” optic nerve pallor was seen only in the eldest affected brother. Examination of the posterior pole revealed the most striking features where a progressively more severe degenerate appearance was seen. In the 6 year old child, mild perifoveal atrophic changes were noted while in the 21 year old increasing pigmentary disturbance was noted (Fig 2). In the 25 year old a definite atrophic region surrounded by pigmentary changes were seen (Fig 3), while in the 30 year old an even more marked staphylomatous region of atrophy and pigmentary change was seen (Fig 4).
Similarly in the retinal periphery progressive changes were seen. Extensive white dots at the level of the retinal pigment epithelium (RPE) were seen in the 6 year old, and these appeared to take a larger rounder appearance with a grey-green hue in the three elder brothers.
DISCUSSION
This report presents the progressive features of LCA seen within a single pedigree arising from a mutation in the LCA5 gene. In this family, progression with age is seen with the development of nystagmus, vitreous opacity, peripheral retinal dots and, most strikingly, the macular staphylomatous appearance. However, despite this progressive picture the visual acuity is poor from birth.
The progressive nature of the condition is well known from examination of individual cases as well as larger studies of pooled patients in whom clinical heterogeneity is seen when looking across a broad age range. Here we present a unique view into the evolution of this disorder and particularly the progressive retinal changes seen within a single pedigree. As the affected individuals are all from the same consanguineous family we assume they have the same underlying defect. We acknowledge that environmental factors may also have had a role in the pathogenesis, but in the cases presented a strong correlation between severity of ocular fundus signs and age is demonstrated. Although not all the retinal dystrophies involve retinal degeneration, the end point of the majority of these diseases is photoreceptor cell death. Animal models suggest a mechanism of death by an apoptotic pathway and not a direct consequence of the biochemical abnormality.19 So, despite many different patterns of pigmentary or atrophic fundus changes, they are the result of degeneration by a single pathway. The process by which these particular patterns arise is poorly understood.
This pedigree describes changes arising from a specific mutation in a single gene. In the field of retinal dystrophies, allelic variation is common resulting in different diseases arising from different mutations within a single gene. This is highlighted by the only other report of Leber’s congenital amaurosis10 caused by a mutation at the LCA5 locus. This report was of disease in a different population, the “Old River Brethren,” who presumably have a different mutation in this gene. The fundal appearance in that pedigree was reported to be normal for most, with progressive peripheral retinal mottling.
In contrast, LCA with CRB1 mutations have been noticed to have two fairly consistent phenotypic features: the relatively early appearance of white spots and pigment clumps and the presence of moderate to high hyperopia.7 Mutations in the GUCY2D and RPE65 genes both give a normal fundal appearance at birth followed by a “salt and pepper” appearance despite very different functional outcomes.20
In the future we can expect to see extensive databases, which provide us with projected pattern of disease progress for specific mutations in individual genes, with essentially each variation being a specific disease process.
Acknowledgments
The authors gratefully acknowledge the Wellcome Trust who funded this research (award 061682/dcp).
REFERENCES
- 1.Taylor DS. Paediatric ophthalmology. 2nd ed. Oxford: Blackwell Science 1997::561–3.
- 2.Leber T. Ueber Retinitis Pigmentosa Und Angeborene Amaurose. Arch Ophthalmol 1869;15:1–25. [Google Scholar]
- 3.Lambert SR, Sherman S, Taylor D, et al. Concordance and recessive inheritance of Leber’s congenital amaurosis. Am J Med Genet 1993;46:275–7. [DOI] [PubMed] [Google Scholar]
- 4.Schappert-Kimmijser J, Henkes HE,Van den Bosch J. Amaurosis congenita (Leber). Arch Ophthalmol 1959;61:211–18. [DOI] [PubMed] [Google Scholar]
- 5.Waardenburg PJ, Schappret-Kimmijser J. On various recessive biotypes of Leber’s congenital amaurosis. Acta Ophthalmol 1963;41:317–20. [DOI] [PubMed] [Google Scholar]
- 6.Marlens F, Bareil C, Griffoin JM, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet 1996;14:415–20. [DOI] [PubMed] [Google Scholar]
- 7.Lotery AJ, Jacobson SG, Fishman GA, et al. Mutations in the CRB1 gene cause Leber’s congenital amaurosis. Arch Ophthalmol 2001;119:415–20. [DOI] [PubMed] [Google Scholar]
- 8.Dryja TP, Adams SM, Grisby JL, et al. Null RPGRIP 1 alleles in patients with Leber’s congenital amaurosis. Am J Human Genet 2001;68:1295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrault I, Rozet JM, Calvas P, et al. Retinal specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nat Genet 1996;14:461–4. [DOI] [PubMed] [Google Scholar]
- 10.Sohocki MM, Browne SJ, Sullivan LS, et al. Mutations in a new photoreceptor-pineal gene on 17p cause Leber’s congenital amaurosis. Nat Genet 2000;24:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freund CL, Wang QL, Chen S, et al. De novo mutations in the CRX homeobox gene associated with Leber’s congenital amaurosis. Nat Genet 1998;18:311–12. [DOI] [PubMed] [Google Scholar]
- 12.Dharmaraj S, Li Y, Robitaille JM, et al. A novel locus for Leber congenital amaurosis on chromosome 6q. Am J Hum Genet 2000;66:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockton DW, Lewis RA, Aboud EB, et al. A novel locus for Leber congenital amaurosis on chromosome 14q24. Hum Genet 1998;103:328–33. [DOI] [PubMed] [Google Scholar]
- 14.Rozet JM, Perrault I, Gerber S, et al. Is guanylate cyclase activating protein (GCAP 30) the fourth gene for LCA? Invest Ophthalmol Vis Sci suppl 2000;abstract 889.
- 15.http:/www.sph.uth.tmc.edu/Retnet/home.htm.
- 16.Lotery AJ, Namperumalsamy P, Jacobson SG, et al. Mutation analysis of three genes in patients with Leber’s congenital amaurosis. Arch Ophthalmol 2000;118:538–43. [DOI] [PubMed] [Google Scholar]
- 17.Smith D, Oestreicher J, Musarella M. Clinical spectrum of Leber’s congenital amaurosis in the second to fourth decades of life. Ophthalmology 1990;97:1156–61. [DOI] [PubMed] [Google Scholar]
- 18.Heher KL, Trabousli EI, Maumenee IH. The natural history of Leber’s congenital amaurosis. Ophthalmology 1992;99:241–5. [DOI] [PubMed] [Google Scholar]
- 19.Travis GH. Mechanisms of cell death in inherited retinal dystrophies. Am J Hum Genet 1998;62:503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrault I, Rozet J, Gerber S, Ghazi I, et al. Leber’s congenital amaurosis. Mol Genet Metab 1999;68:100–208. [DOI] [PubMed] [Google Scholar]