Abstract
Background: Behçet's disease is a multisystem vasculitis of unknown origin. Standard treatment mainly comprises systemic immunosuppressive agents. Ocular involvement, mostly posterior uveitis with retinal vasculitis, leads to blindness in 20–50% of the involved eyes within 5 years. The efficacy of interferon alfa-2a was studied in patients with sight threatening posterior uveitis or retinal vasculitis.
Methods: 50 patients were included in this open, non-randomised, uncontrolled prospective study. Recombinant human interferon alfa-2a (rhIFNα-2a) was applied at a dose of 6 million units subcutaneously daily. Dose reduction was performed according to a decision tree until discontinuation. Disease activity was evaluated every 2 weeks by the Behçet's disease activity scoring system and the uveitis scoring system.
Results: Response rate of the ocular manifestations was 92% (three non-responder, one incomplete response). Mean visual acuity rose significantly from 0.56 to 0.84 at week 24 (p<0.0001). Posterior uveitis score of the affected eyes fell by 46% every week (p<0.001). Remission of retinal inflammation was achieved by week 24. Mean Behçet's disease activity score fell from 5.8 to 3.3 at week 24 and further to 2.8 at week 52. After a mean observation period of 36.4 months (range 12–72), 20 patients (40%) are off treatment and disease free for 7–58 months (mean 29.5). In the other patients maintenance IFN dosage is three million units three times weekly.
Conclusions: rhIFNα-2a is effective in ocular Behçet's disease, leading to significant improvement of vision and complete remission of ocular vasculitis in the majority of the patients.
Keywords: human recombinant interferon alfa-2α, Behçet's disease, uveitis, retinitis
Behçet's disease is a multisystem vasculitis of unknown origin, which is most prevalent in Mediterranean countries, Asia and the Middle East, which lie along the former “silk route.”1–3 Its main features4,5 are oral and genital aphthous ulcers, skin manifestations such as erythema nodosum, papulopustules, or leucocytoclastic vasculitis, oligoarthritis, peripheral vascular manifestations such as thrombophlebitis, thrombosis, aneurysms, and neurological manifestations. The disease is associated with HLA B51. Ocular manifestations, mostly a bilateral panuveitis running a chronic relapsing course, are present in 60–80% of the patients. In most studies, blindness occurred in 20–50% of the patients within 5 years.6–9 With the increasing use of immunosuppressive agents, ocular prognosis has improved.10 Azathioprine has been shown to maintain visual acuity and prevent development of eye disease,11,12 but in our experience, especially in severe panuveitis and retinal vasculitis, may not act rapidly enough. Cyclosporin A is an effective and rapidly acting drug for eye disease.13–17 However, nephrotoxicity, particularly at doses higher than 5 mg/kg/day, and relapses after cessation of therapy often limit its use. Cytotoxic agents such as cyclophosphamide and chlorambucil are also used but have been less well studied. Colchicine is effective for mucocutaneous and articular manifestations, but only partially effective for posterior uveitis.18 Brief courses of corticosteroids may shorten the duration of the attacks but they are not effective for long term treatment, probably because the dose necessary for maintenance of remission would be very high with unacceptable side effects.
Up to now, interferons have only been used in selected small cohorts with Behçet's disease. A few open studies with up to 20 patients without ocular disease, showed efficacy for interferon alfa (IFN) and in different doses.19–23 Recently, there have been case reports on efficacy in four patients with severe refractory eye disease successfully treated with steroids, immunosuppressants, and IFN in various combinations24–26 and four small open studies with a total of 86 patients, 21 of whom had ocular inflammation.27–33 Eye disease was reported to respond to IFN treatment, but details were not described. A randomised controlled study recently published34 with 135 patients (67 randomised to IFN plus colchicine plus penicillin, previous ocular disease was excluded) unfortunately had to be retracted owing to fabrications with respect to authorship and possibly also the reported data and ethical transgressions.34–37 We treated a patient who had developed Kaposi's sarcoma under triple immunosuppressive therapy for his severe ocular Behçet's disease38 with rhIFNα-2a and were able to induce remission of both diseases. This prompted us to initiate a pilot study on the efficacy of rhIFNα-2a in severe ocular Behçet's disease.31,32 Because of the promising results, it was continued as a four centre, prospective, open, uncontrolled study.
METHODS
Study design
Fifty patients with highly resistant ocular Behçet's disease were included in four participating hospitals from March 1995 to March 2000, the last examination considered for evaluation was performed in March 2001. It was a prerequisite for entering the study for the patients to have active posterior uveitis or panuveitis which had been refractory to at least one conventional immunosuppressive drug (for example, azathioprine or cyclosporin A) or prednisolone in a dose of at least 1 mg/kg bodyweight and/or impossibility to taper the steroids to a maintenance dosage of less than 30 mg prednisolone equivalent per day. This drug had to be given for at least 2 weeks. Forty six patients fulfilled the International Study Group criteria,39 four patients had incomplete Behçet's disease with oral aphthous ulcers and panuveitis with typical occlusive retinal vasculitis and/or hypopyon. The study protocol (adhering to the Declaration of Helsinki) had been approved by the institutional review board of each hospital and the patients had given informed consent. The patients had to be older than 18 years. Exclusion criteria included pregnancy, fertile women without contraception, and patients with additional metabolic, psychiatric, or malignant diseases. Previous therapy with immunosuppressants or other drugs had to be discontinued before the initiation of interferon treatment and systemic glucocorticosteroids had to be reduced to a maximum of 10 mg prednisolone equivalent per day because antagonistic effects were suspected and in order to be sure that the effects observed were exclusively due to IFN treatment and not to high or medium dose steroids or immunosuppressive drugs. Topical non-steroidal antirheumatic drugs and steroids were permitted for anterior uveitis.
Treatment
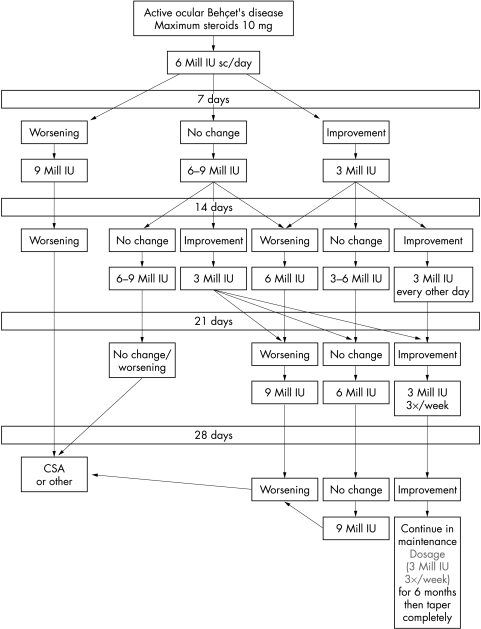
Patients received 6 million international units (IU) of rhIFNα-2a subcutaneously (sc) daily for at least 14 days. Dosage was then adjusted according to a flow chart (Fig 1) with a maximum of 9 million units daily. In three female patients IFN was kept on 3 million IU daily initially because of severe flu-like syndrome. Glucocorticosteroids were reduced to a maximum 10 mg/day within 1–5 days (depending on previous duration of steroid treatment), kept at this dosage until complete remission of ocular disease and tapered to 5 mg later. Prednisolone was completely tapered in case of remission of both ocular and systemic disease.
Figure 1.
Flow chart for interferon dosage. In case of worsening after dose reduction return to last effective dosage.
Immunosuppressive drugs were stopped immediately at least 1 day before initiation of IFN treatment. Paracetamol (three times 500 mg daily) was given on days 1–3 and later in case of fever or arthralgia due to IFN.
Outcome, safety, and side effect measurements
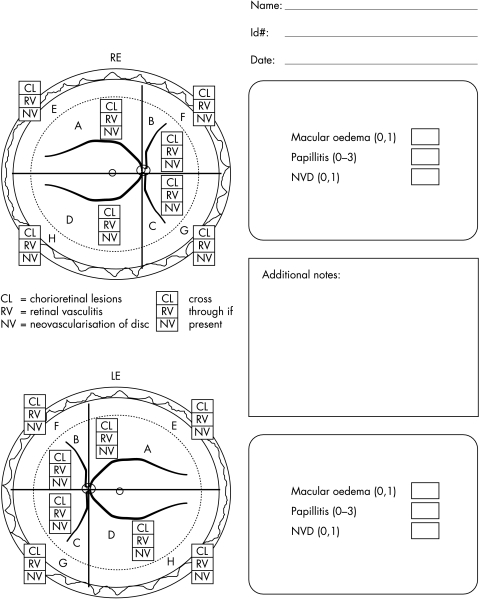
The patients were seen at baseline, daily for 1 week, every 2 weeks for the first 2 months, then every 4 weeks by the same ophthalmologist and rheumatologist. After cessation of IFN treatment, patients were followed up every 8 weeks for 6 months and then every 12 weeks if the course was uneventful. In case of subjective progression/relapse of ocular disease, the patients were asked to present immediately. A complete physical and ophthalmological examination (visual acuity, slit lamp examination, tonometry, and ophthalmoscopy) was performed on each visit. A comparison of European decimal and Snellen chart is provided in Table 1. Ocular inflammation was assessed by the uveitis scoring system,40 which includes visual acuity, cataract progression, inflammatory activity of the anterior and posterior chamber (Fig 2). As the uveitis scoring system evaluates all these parameters separately and the main efficacy variables were posterior uveitis/retinal vasculitis and visual acuity, these two variables were considered for statistical analysis only. The other disease features were assessed by the Behçet's disease activity scoring system.41 Fluorescein angiography was performed at baseline, after 4 weeks and then every 8 weeks for the first 6 months, later every 6 months, and additionally on demand (in case of worsening of ocular symptoms). Laboratory safety parameters (differential blood count, platelets, coagulation values, liver enzymes, creatinine) were evaluated in parallel with the clinical examinations. Thyroid hormones were measured by enzyme linked immunosorbent assay (ELISA) every 4 weeks. At baseline and every 6 months, interferon antibodies were measured by ELISA (Bender MedSystems, Vienna, Austria).
Table 1.
Comparison of European decimals to Snellen equivalent for measurement of visual acuity
| Snellen equivalent | ||
| European decimal | 5 metres | 1 metre |
| 0.025 | 5/200 | |
| 0.03 | 5/160 | |
| 0.04 | 5/125 | |
| 0.05 | 5/100 | |
| 0.06 | 5/80 | |
| 0.08 | 5/63 | |
| 0.1 | 20/200 | 5/50 |
| 0.125 | 20/160 | 5/40 |
| 0.16 | 20/125 | 5/32 |
| 0.2 | 20/100 | 5/25 |
| 0.25 | 20/80 | 5/20 |
| 0.32 | 20/63 | 5/16 |
| 0.4 | 20/50 | 5/12.5 |
| 0.5 | 20/40 | 5/10 |
| 0.63 | 20/32 | |
| 0.8 | 20/25 | |
| 1.0 | 20/20 | |
| 1.25 | 20/16 | |
Figure 2.
Scoring sheet for posterior uveitits score (BenEzra et al40).
Antinuclear antibodies and thyroid antibodies were evaluated by indirect immunofluorescence on Hep-2 cells and by ELISA at baseline and every 4 months. In case of positive results, the antibodies were further specified by immunodiffusion or western blot. HLA-typing was performed by oligonucleotide typing at baseline. Side effects were documented by the patients in a diary.
Statistical analysis
If visual acuity was reduced below 0.063 the following standard was used for statistical evaluation: letter recognition at 1 metre = 0.02, counting fingers = 0.005, hand movements = 0.002, light perception = 0.001. Time trends were fitted as simple curves using linear analysis of covariance (ANCOVA). Individual curves had different intercepts for each patient (random effect estimated by restricted maximum likelihood (REML)). IFN dose at the time of observation was included in all models along with its interaction with the respective time variable. As posterior uveitis score and visual acuity were observed for each eye, the status of the respective eye at the start of treatment as affected or unaffected had to be taken into account when estimating the improvement over time. For the Behçet's disease activity score (BD score) such a bisection was not necessary. Residuals' normality and homoscedasticity were assessed by quantile-quantile plot (QQ plot) and residuals by predicted plot, respectively. Quality of fit was recorded as adjusted coefficient of determination (adj R2). Time to improvement of posterior uveitis score and BD score was defined as time to 50% below starting levels of the respective measurements. This was taken from the individual record and used to estimate the median for the patient population with its 95% confidence interval. Similarly, time to remission was defined as time to the first observation of the scores being 0 and estimated likewise.
RESULTS
The characteristics of the 50 patients are shown in Tables 2 and 3. All patients except three had previously received high dose (≥1.5 mg/kg bodyweight) steroids, which had not been effective after 2 weeks or later on could not be tapered to a reasonable maintenance dosage because of ocular relapses. Twenty six patients (52%) had had other, in some cases several, immunosuppressive therapies in various combinations (cyclosporin A (n = 20), azathioprine (n = 13), cyclophosphamide (n = 4), methotrexate (n = 4), colchicine (n = 2), chlorambucil (n = 1), mycophenolate mofetil (n = 1)), all of which had been ineffective for their ocular disease. Six of the patients (Nos 1, 2, 3, 5, 7, 30) have already been included in a previously published pilot study.31 Patient 7 is the patient with Kaposi's sarcoma. He had stopped IFN treatment in complete remission on his own in April 1997 and presented with a relapse of his posterior uveitis 6 weeks later in June 1997. He then was included in the present open study. One patient originally included in the first study withdrew her consent for further publication of her data and consequently was not included in the present evaluation. Another 27 patients have additionally been included in the second part of the pilot study (patients 4, 6, 8, 9, 10, 14, 15, 17–25, 31, 33, 36, 37, 40–46).32 At baseline, 49 patients had been treated with systemic corticosteroids (>10 mg prednisolone equivalent) for their current ocular inflammation. This was reduced to 10 mg within 1–5 days (depending on the duration of previous steroid treatment) before initiation of IFN treatment. HLA B51 was positive in 42 patients (84%). Mean observation period is 36.4 months (range 12–72 months). BD scores could be obtained in 49 patients. Of 100 eyes, 79 were affected (posterior uveitis score ≥1).
Table 2.
Patient characteristics, disease manifestations
| Macular oedema | |||||||||||
| No | Sex | DOB | HLA-B51 | Nat | dxBD | pmBD | Manifestation | pm eye | Eye manifestation | od | os |
| 1 | F | 1963 | pos | G | 1994 | 1992 | ou, gu, j, skin, eye | 1995 | panuveitis od | x | |
| 2 | M | 1973 | neg | Ger | 1995 | 1989 | ou, gu, skin, eye | 1995 | panuveitis bil, occl vasc od | x | |
| 3 | M | 1957 | pos | I | 1990 | 1989 | ou, gu, j, eye | 1989 | panuveitis, occl vasc os | x | |
| 4 | F | 1956 | neg | J | 1995 | 1994 | ou, gu, skin, eye | 1990 | panuveitis od | x | |
| 5 | F | 1969 | neg | T | 1991 | 1989 | ou, skin, cns, eye | 1994 | panuveitis os | ||
| 6 | M | 1953 | pos | Ger | 1989 | 1990 | ou, skin, j, eye | 1991 | panuveitis od | x | |
| 7 | M | 1965 | pos | T | 1989 | 1983 | ou, eye, epid | 1989 | Panuveitis os | x | |
| 8 | M | 1965 | pos | Ger | 1992 | 1987 | ou, gu, eye, skin | 1992 | panuveitis od | x | |
| 9 | M | 1960 | pos | T | 1996 | 1994 | ou, gu, j, eye, skin, gi | 1996 | panuveitis, bil | x | x |
| 10 | M | 1969 | neg | Alb | 1997 | 1994 | ou, j, eye | 1991 | panuveitis occl vasc os | x | |
| 11 | M | 1965 | pos | T | 1995 | 1994 | ou, skin, eye | 1995 | panuveitis, hypopyon bil | x | x |
| 12 | M | 1969 | pos | T | 1989 | 1989 | ou, gu, skin j, thr, epid, eye | 1996 | panuveitis, bil | x | x |
| 13 | M | 1963 | pos | T | 1994 | 1990 | ou, skin epid, eye | 1996 | panuveitis os | x | |
| 14 | F | 1945 | pos | Ger | 1997 | 1981 | ou, j, skin, eye | 1985 | panuveitis bil | x | x |
| 15 | F | 1961 | pos | T | 1994 | 1994 | ou, skin, eye | 1995 | panuveitis bil | x | x |
| 16 | F | 1957 | pos | T | 1997 | 1993 | ou, skin, j, eye | 1996 | panuveitis bil | x | x |
| 17 | F | 1976 | pos | Ger | 1996 | 1996 | ou, gu, skin, j, eye | 1996 | panuveitis bil, ret vasc os | x | x |
| 18 | M | 1971 | pos | Ger | 1997 | 1996 | ou, cns, thr, eye | 1997 | panuveitis os | ||
| 19 | F | 1958 | neg | Ger | 1990 | 1978 | ou, gu, skin, eye | 1987 | posterior uveitis, occl vasc bil | x | x |
| 20 | M | 1976 | pos | T | 1998 | 1999 | ou, eye | 1998 | okklus vasc, panuveitis bil | x | x |
| 21 | M | 1956 | pos | Iran | 1985 | 1985 | ou, skin, j, eye | 1985 | panuveitis bil/occlus vasc od/papillitis os | ||
| 22 | M | 1978 | pos | J | 1999 | 1997 | ou, skin, eye | 1997 | panuveitis, bil | x | x |
| 23 | F | 1964 | pos | T | 1999 | 1999 | ou, gu, skin, eye | 1999 | panuveitis bil | ||
| 24 | F | 1964 | pos | T | 1992 | 1992 | ou, gu, skin eye | 1992 | panuveitis occl vasc od | x | |
| 25 | F | 1970 | pos | Ger | 1998 | 1998 | ou, gu, skin eye | 1998 | posterior uveitis bil | x | |
| 26 | M | 1973 | pos | T | 1994 | 1994 | ou, gu, skin, eye | 1994 | panuveitis, hypopyon bil, | x | x |
| 27 | F | 1972 | neg | T | 1996 | 1995 | ou, gu, skin, eye | 1996 | panuveitis, hypopyon bil, | x | x |
| 28 | M | 1963 | pos | T | 1991 | 1983 | ou, gu, j, epid, eye | 1991 | panuveitis, od | ||
| 29 | M | 1974 | pos | T | 1995 | 1994 | ou, eye | 1994 | panuveitis, hypopyon od | x | |
| 30 | M | 1958 | pos | T | 1984 | 1984 | ou, j, gi, eye | 1984 | occl vasc od | x | |
| 31 | F | 1972 | pos | Alb | 1995 | 1995 | ou, skin, j, thr, eye | 1997 | panuveitis bil | ||
| 32 | M | 1966 | pos | T | 1995 | 1996 | ou, skin, eye | 1996 | panuveitis od | ||
| 33 | M | 1974 | pos | T | 1997 | 1996 | ou, skin, j, epid, eye | 1996 | panuveitis bil | x | x |
| 34 | M | 1965 | neg | Ger | 1996 | 1990 | ou, gu, j, skin, eye | 1997 | uveitis post ret vasc od | ||
| 35 | M | 1967 | pos | T | 1994 | 1993 | ou, gu, skin, j, eye | 1994 | post uveits bil ret vasc, hypopyon bil | x | x |
| 36 | M | 1955 | pos | T | 1989 | 1989 | ou, gu, j, skin, eye | 1989 | panuveitis bil | x | x |
| 37 | M | 1949 | pos | T | 1998 | 1992 | ou, skin, eye epid, | 1998 | panuveitis bil | x | x |
| 38 | M | 1970 | neg | T | 1997 | 1990 | ou, gu, skin, j, eve | 1997 | panuveitis bil | x | |
| 39 | M | 1968 | pos | Leb | 1999 | 1997 | ou, gu, j, eye | 1997 | panuveitis bil | ||
| 40 | M | 1972 | pos | T | 1996 | 1999 | ou, gu, skin, eye | 1999 | panuveitis bil, occl vasc os (papillophlebitis) | x | |
| 41 | M | 1972 | pos | T | 1998 | 1997 | ou, gu, skin, j, epid, eye | 1998 | paunuveitis bil (occl vasc) | x | x |
| 42 | M | 1972 | pos | Ger | 1998 | 1999 | ou, skin, eye | 1998 | retinal vasculitis bil | ||
| 43 | M | 1962 | pos | Mor | 1998 | 1998 | ou, skin, j, eye | 1998 | panuveitis, ret vasc bil | x | x |
| 44 | M | 1976 | neg | T | 1996 | 1996 | ou, skin, eye | 1996 | panuveitis bil | x | |
| 45 | M | 1977 | pos | T | 1999 | 1999 | ou, gu, skin, eye | 1999 | panuveitis bil | x | |
| 46 | M | 1974 | pos | T | 1996 | 1991 | ou, gu, skin, eye | 1996 | panuveitis bil | x | x |
| 47 | M | 1960 | pos | T | 1999 | 1999 | ou, skin, thrphleb), eye | 1991 | posterior uveitis os | x | |
| 48 | M | 1961 | pos | T | 1992 | 1992 | ou, gu, j, eye | 1992 | posterior uveitis os | ||
| 49 | M | 1955 | pos | Ger | 1997 | 1992 | ou, j, eye, skin | 1997 | panuveitis, retinal vasculitis bil | x | x |
| 50 | F | 1972 | pos | T | 1997 | 1997 | ou, gu, j, eye, cns | 1997 | episkcleriis, retinal vasculitis od | ||
No = number; abbr = abbreviation; DOB = date of birth; nat = nationality; dxBD = diagnosis of BD (year); pmBD = primary manifestation (year); nd = not done; pos = positive; neg = negative; pm = primary manifestation; ou = oral ulcers; gu = genital ulcers; j = joint (arthritis); epid = epididymitis; thr = thrombosis; thrphleb = thrombophlebitis; od = right eye; os = left eye; bil = bilateral; Ster = steroids; CSA = cyclosporin A; AZA = azathioprine; MTX = methotrexate; Cy = cyclophosphamide; Chloramb = chlorambucil; MMF = mycophenolate mofetil; Col = colchicine; CR = complete remission; noncompl = non-compliance; T = Turkish; Ger = German; I = Italian; G = Greek; Mor = Moroccan; Leb = Lebanese; Alb = Albanian.
Table 3.
Patient characteristics, previous therapies, and course of IFN therapy
| No | Previous therapy | Start IFN | Stop IFN | Reason stop IFN | Number of ocular relapses |
| 1 | Ster, CSA | Mar 95 | Jun 97 | CR | 0 |
| 2 | Ster, AZA | Nov 95 | Sep 00 | CR | 2 |
| 3 | Ster, Col, IFNγ | Nov 95 | 0 | ||
| 4 | Ster, AZA | Dec 96 | Nov 99 | CR | 0 |
| 5 | Ster | Aug 96 | May 97 | CR | 0 |
| 6 | Ster, MTX | Oct 96 | 15 | ||
| 7 | Ster, CSA, AZA | Sep 96 | Jan 98 | CR | 1 |
| 8 | Ster, CSA | Mar 97 | Jan 00 | CR | 0 |
| 9 | Ster, MTX | May 97 | Jul 98 | CR | 0 |
| 10 | Ster, CSA | May 97 | Jul 98 | CR | 0 |
| 11 | Ster, CSA | Mar 97 | 0 | ||
| 12 | Ster, AZA | Apr 97 | 0 | ||
| 13 | Ster | Mar 97 | Mar 98 | CR | 0 |
| 14 | Ster | Nov 97 | Feb 99 | CR | 0 |
| 15 | Ster | Nov 97 | Dec 98 | CR | 0 |
| 16 | Ster | Oct 97 | 0 | ||
| 17 | Ster, CSA | Nov 98 | Nov 99 | noncompl (CR), AZA ineff | 0 |
| 18 | Ster, AZA | Jun 97 | May 98 | CR (eye), lost to follow up | |
| 19 | Ster, AZA, CSA | Dec 95 | Jan 97 | Ineff | |
| 20 | Ster, MMF | Jul 99 | 0 | ||
| 21 | Ster | Apr 99 | 0 | ||
| 22 | Ster, CSA | May 99 | 0 | ||
| 23 | none | May 99 | Aug 00 | CR (noncompl), lost to follow-up | |
| 24 | Ster, AZA, CSA | Dec 98 | 0 | ||
| 25 | Ster, CSA | Mar 99 | 0 | ||
| 26 | Ster, CSA, Cy, AZA | May 97 | Feb 98 | ineff (Cy, ineff) | |
| 27 | Ster, CSA, Cy, | Jun 97 | Dec 97 | side eff (CR), (Aza ineff) | |
| 28 | Ster, CSA | Oct 97 | Oct 98 | CR | 0 |
| 29 | Ster, CSA, Cy | Dec 97 | May 98 | ineff (Cy, Aza ineff) | |
| 30 | Ster, CSA | Aug 96 | Feb 97 | side eff (PR) | 0 |
| 31 | Ster Col | Dec 96 | Jun 97 | CR, lost to follow up | 0 |
| 32 | Ster, AZA | Jan 96 | Jun 96 | CR | 0 |
| 33 | Ster AZA | Aug 97 | Aug 98 | CR, died in car acc | |
| 34 | none | Sep 96 | Dec 97 | CR | 0 |
| 35 | Ster, CSA | Oct 96 | Sep 97 | add IFNγ | |
| 36 | Ster, AZA, CSA | Jun 99 | 0 | ||
| 37 | Ster, AZA | Apr 99 | Jan 00 | CR | 0 |
| 38 | Ster | Aug 99 | 0 | ||
| 39 | Ster, AZA | Mar 99 | Jun 99 | CR, pathergy pos | 0 |
| 40 | Ster | Oct 99 | 1 | ||
| 41 | Ster, CSA | Aug 99 | 1 | ||
| 42 | Ster, CSA | Sep 99 | 0 | ||
| 43 | Ster | Jan 00 | 0 | ||
| 44 | none | Jan 00 | 0 | ||
| 45 | Ster, AZA | Feb 00 | 0 | ||
| 46 | Ster, MTX, CSA | Jan 00 | 0 | ||
| 47 | Ster, CSA, Chloramb | Mar 00 | 0 | ||
| 48 | Ster | Aug 97 | 0 | ||
| 49 | Ster, MTX, Cy | Dec 98 | 0 | ||
| 50 | Ster | May 98 | Jan 00 | CR | 1 |
Ster = steroids; CSA = cyclosporin A; AZA = azathioprine; MTX = methotrexate; Cy = cyclophosphamide; Chloramb = chlorambucil; MMF = mycophenolate mofetil; Col = colchicine; CR = complete remission; noncompl = noncompliance.
Response of ocular disease
Visual acuity
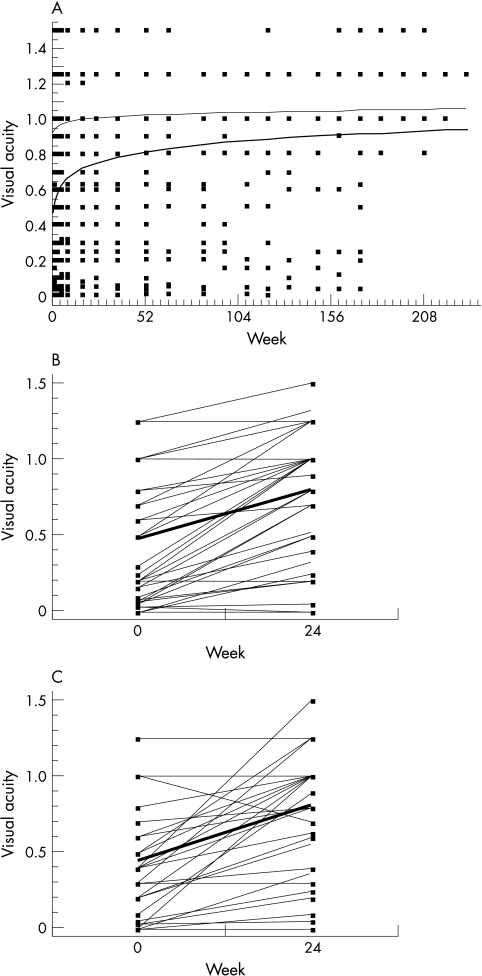
Visual acuity was regressed on the logarithm of weeks under therapy plus one. Mean visual acuity (VA) in the affected eyes (n = 79) rose significantly (slope p<0.0001) from 0.56 (SD 0.37) at week 0 to 0.84 at week 24 and remained stable at week 108 (37 eyes) (SD 0.40) (Fig 3). In the affected eyes, it improved by 0.13 in the first week, but by less than 0.04 in the unaffected eyes. The effect of IFN dose could be neglected, as neither its own effect nor the interaction with the time variable were significant (p = 0.8 and 0.6, respectively). Regarding the affected eyes at weeks 0 and 24 (n = 73, data for six eyes were not available at that exact time point), visual acuity improved (defined as improvement on the scale of at least two steps) in 75.3% (n = 55), remained stable in 22% (n = 16), and worsened in 2.7% (n = 2). The increase of visual acuity for the right eyes was 0.33, for the left eyes 0.36 (Fig 3B and C). Three eyes were and remained blind. Altogether, seven eyes (7%) had a final visual acuity below 0.1, which in each case had remained unchanged in comparison with baseline. In all these cases, the loss of vision had been irreversible because of macular scars and/or retinal ischaemia as a consequence of longstanding refractory retinal vasculitis.
Figure 3.
(A) Mean visual acuity by week of IFN treatment for the affected (thick line) and the unaffected (thin line) eyes. (B) Visual acuity of the affected right eyes at weeks 0 and 24 (n=35), thick line: mean. (C) Visual acuity of the affected left eyes at weeks 0 and 24 (n=37), thick line: mean.
Posterior uveitis score
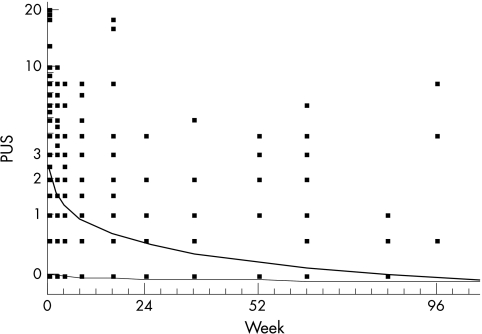
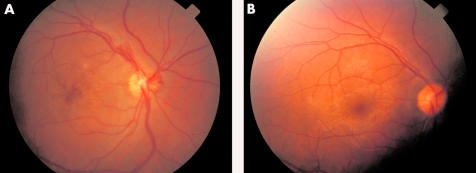
Posterior uveitis scores were transformed to natural logarithms of score plus one in 100 eyes, 79 of which were affected. There is no significant slope for the unaffected eyes (p = 0.5), but scores of the affected eyes were reduced by 46% weekly (p<0.001, Fig 4). Applying the Bonferroni-Holm procedure in order to adjust for multiple testing renders the time-dose interaction insignificant. Fitted posterior uveitis score fell from 3.5 (week 0) to 0.4 at week 24. Remission (defined as a posterior uveitis score 0, remission of retinal inflammation) in all affected eyes of the responders (n = 71) was reached by week 24. The median time to remission was 4 weeks (95% confidence interval week 2 to week 4). Retinal infiltrates resolved after 2–3 weeks in all patients, and active vascular sheathing disappeared after 4–6 weeks, as did vitreous opacity. In two patients (Nos 2 and 3) an acute venous branch occlusion was present and the vessels were reperfused with only small areas of non-perfusion persisting in the periphery, as proved by fluorescein angiography. Macular oedema was seen in the fluorescein angiograms in 58 eyes and disappeared without additional therapy (for example, acetazolamide) in all cases. As an example, Figure 5 shows the retinal changes of patient No 41 before and after 6 months of IFN treatment.
Figure 4.
Geometric mean of posterior uveitis score (PUS) by IFN treatment over time for affected eyes (thick line) and unaffected eyes (thin line).
Figure 5.
Patient No 40 (A) before IFN treatment. Right eye, oedema of optic disc and macula, macular bleeding, neovascularisation of the optic disc, VA 0.2; (B) 6 months later, right eye VA 1.0, normal fundus.
Anterior uveitis score
At baseline, additional inflammation of the anterior chamber was present in 66 eyes. Median anterior uveitis score at initiation of IFN treatment of all affected eyes (n = 79, anterior uveitis score ≥1) was 1 (range 0–10), it fell to a median of 0 (range 0–3) at week 24 and to a median of 0 (range 0–3.5) at week 52, respectively. In general, 2 weeks after the first IFN application, the anterior chamber was free of inflammatory cells. As mentioned above, non-steroidal antirheumatic and prednisolone eye drops were given locally in case of anterior uveitis, thus it is not possible to decide whether the improvement in the anterior chamber of the eye was due to IFN or if it also would have been achieved with the local standard treatment alone.
Response of extraocular manifestations
Before treatment all 50 patients had typical oral aphthous ulcers (100%), 37 patients had cutaneous manifestations (74%), 26 genital ulcers (50.2%), 25 arthritis (50%), three patients had vascular manifestations (6%), three CNS manifestations (6%), and two gastrointestinal ulcerations (4%). Mean Behçet's disease activity score fell from 5.8 to 3.3 at week 24 and further to 2.8 at week 52.
Dropouts and compliance
In addition to the four patients who did not or only partially respond to IFN treatment, two patients who were in complete remission were switched to azathioprine (AZA) because of hair loss (Nos 17 and 27), in both of whom it was not effective in controlling ocular inflammation. One patient (No 30) stopped IFN in complete remission because of diarrhea and is now being treated with low dose steroids. Three patients (Nos 18, 23, and 31) dropped out because of non-compliance in complete remission (they prematurely stopped the treatment on their own) and were lost to follow up later. These patients were statistically analysed as responders.
Non-responder, overall response, frequency, and severity of relapses
Three patients did not respond to dosages below or equal to 9 million units rhIFNα-2a; they were regarded as non-responders and switched to other treatments (patient No 19 cyclosporin plus azathioprine, patient No 26 cyclophosphamide, patient No 30 cyclophosphamide and azathioprine). All experienced progressive disease and loss of vision irrespective of the aggressive immunosuppression. One patient (No 34) received a combination of IFNγ and IFNα according to a previous case report,42 because of relapses of his panuveitis with IFNα dosages below 6 million IU daily. He entered complete remission with this combination and IFNα was discontinued, whereas IFNγ currently is being taken at a maintenance dose of 3 million IU three times per week. Thus, the overall response of ocular manifestations was 92% (46/50).
In 41 patients (82%), no ocular relapses occurred. rhIFNα-2a could be discontinued in 20 patients (40%) and tapered to the maintenance dose of 3 million IU three times weekly in 27 (54%). In six responders (12%), relapses with worsening of oral aphthous ulcers, cutaneous and articular manifestations, and minor ocular inflammation (mostly mild anterior uveitis) occurred during dose reduction below 3 million units three times weekly, which in all cases responded to an increase of rhIFNα-2a treatment according to the flow chart (Fig 1). The mean number of relapses in the responders was 0.44 (range 0–15); these relapses are mainly because of patient No 6, who experienced minor ocular relapses (isolated retinal infiltrates in the periphery) with each dose reduction (15 at all) and at present is in remission on the maintenance dose of 3 million IU three times weekly.
Adverse effects
The adverse effects are summarised in Table 4. Fever and arthralgia occurred in all patients during the first week of treatment with rhIFNα-2a. Reddening at the site of injection was also observed in all patients, independent of IFN dosage. Leucopenia (2000–3000 × 106/l) was observed in 20 patients (40%) with doses of 3 million units daily or above. Hair loss which improved with dose reduction, was observed in 12 (24%) patients and was the cause for stopping the otherwise effective treatment with rhIFNα-2a in two of them (Nos 17 and 27). Depression was observed in four patients (8%), but improved in all but one after 2 weeks or after dose reduction of IFN. The remaining individual (No 15) was treated successfully with amitriptyline. In two patients (Nos 6 and 9), a pre-existing psoriasis worsened. In patient No 6 no treatment was necessary, in the other (No 9), generalised psoriasis occurred and topical therapy with psoralen and ultraviolet B light was applied. Itching was another side effect observed in 10 patients (20%) on 6 million IU but not at lower doses. Five patients (10%) developed fibromyalgia during IFN treatment, which also improved with dose reduction. One patient experienced an increased number of epileptic seizures on IFNα-2a which were primarily due to his CNS vasculitis. These were successfully treated with valproinic acid.
Table 4.
Side effects of IFN
| Symptom | No | % |
| Flu-like syndrome | 50 | 100 |
| Reddening at site of injection | 50 | 100 |
| Leucopenia | 20 | 40 |
| Alopecia | 12 | 24 |
| Itching | 10 | 20 |
| Fibromyalgia | 5 | 10 |
| Depression | 4 | 8 |
| Worsening of psoriasis | 2 | 4 |
| Thyroiditis | 2 | 4 |
| Worsening of seizures | 1 | 2 |
| Autoantibodies | ||
| ANA | 8 (1 dsDNA) | 16 |
| Thyroid | 3 | 6 |
Flu-like syndrome is fatigue, headache, arthralgia, fever.
ANA = antinuclear antibodies.
Autoimmune phenomena
In eight patients (16%), antinuclear antibodies, in one of them (No 5), dsDNA antibodies developed. In all cases, there was no sign of clinical connective tissue disease except fibromyalgia in one patient (No 15). Antithyroid antibodies were observed in three patients (Nos 2, 8, and 15). Patients 8 and 15 developed Hashimoto thyroiditis with subsequent hypothyroidism. Anti-IFN antibodies remained negative in all patients during the follow up.
Maintenance dosage/discontinuation of therapy
In 20 patients (40%) therapy was discontinued after a mean period of 16.4 months (range 3–58) without relapse of ocular symptoms. The mean observation period after discontinuation of IFNγ in 17 of these 20 patients (those who were not lost to follow up or died later) is 29.5 months (range 7–58). In four of the patients (Nos 1, 2, 3, and 9) oral aphthous ulcers and cutaneous symptoms worsened after discontinuation of IFN but they were successfully treated with colchicine. In the other responders, IFN was reduced to 3 million units three times weekly, but could not be tapered off completely. Steroids were completely tapered off in 81% of the responders. Mean prednisolone dosage of the responders at baseline was 97 mg (range 0–1000), at week 52 it had been reduced to a mean of 2 mg (range 0–10).
DISCUSSION
Interferon alfa-2a was effective (response rate 92%) and rapidly acting (time to response 2–4 weeks) for the treatment of severe ocular Behçet's disease. No primarily unaffected eyes became involved during the observation period. The mean number of relapses was very low (0.44 during a mean observation period of 36.4 months). For cyclosporin A, the response rates reported in small, uncontrolled series and two controlled studies were between 80% and 91%.13–17 Of note, doses of 5–10 mg/kg body weight were used, side effects were frequent, and the number of relapses during dose reduction was high. Time to response with cyclosporin A is not reported in these studies. Cyclosporin A was significantly more effective than cyclophosphamide (1000 mg intravenous pulses monthly)16 or colchicine (response rate 33%).14 For azathioprine, no such data are available, but in one large placebo controlled trial azathioprine treatment led to significantly better visual acuity than placebo after 24 months.11 Frequency of ocular attacks could be significantly reduced with both drugs, with a mean of six per year for cyclosporin A.16 Although the different study designs hamper the comparison of the data reported in the literature, rhIFNα-2a, as studied in this report, seems to have at least the same response rates as high dose cyclosporin A, but it may lead to a more stable response with a lower frequency of ocular relapses. With rhIFNα-2a only 7% of all eyes (9% of the affected eyes) at the end of the observation period had a visual acuity below 0.1, which may be better than the data reported in the literature (20–50% after 5 years), although the results of the re-evaluation of the IFN treated patients after 5 years must be awaited. Furthermore, discontinuation of rhIFNα-2a without ocular relapses was possible in 40% of the patients, which to our knowledge has not been reported for the immunosuppressive drugs.
Side effects of rhIFNα-2a were frequent but, except for hypothyroidism, dose dependent and reversible. The development of autoimmune phenomena during treatment with IFN may be a major concern. These have been observed in patients with chronic hepatitis C and malignant, especially haematological, diseases43 treated with IFN. Mostly, thyroid antibodies and antinuclear antibodies (ANA) have been described, which were rarely associated with clinically overt thyroid or connective tissue disease.44–47 In the present study, autoantibodies developed in 16% of the patients. Thyroid antibodies were detected in three patients (6%), with thyroiditis in two. ANA were not associated with clinically overt autoimmune phenomena in our study, although fibromyalgia in connection with the appearance of autoantibodies, especially dsDNA, may be regarded as an early sign of development of connective tissue disease, because fibromyalgia often is associated with connective tissue diseases, especially with systemic lupus erythematosus.48 The worsening or even de novo development of psoriasis has also been described with the use of IFN in the literature49 and was observed in two of our patients (4%), who were the only ones with a history of psoriasis. Other, less frequent, side effects described in the literature are an interferon induced retinopathy with retinal infiltrates similar to those occurring in Behçet's disease itself, which has mainly been observed in patients with hepatitis C,50,51 and an anterior ischaemic optic neuropathy.52 The development of cutaneous leucocytoclastic vasculitis and even Behçet's disease itself during IFN has been described.53–55 We did not observe any of these. In contrast, retinal infiltrates and even macular oedema disappeared during treatment with rhIFNα-2a, as did cutaneous vasculitis.
The mode of action of rhIFNα in Behçet's disease is still unknown. It has many immunomodulatory effects, such as enhancement of HLA class I antigen expression on lymphoid cells and T and NK cell cytotoxicity, and it diverts the T cell response in the direction of Th1.56 All these effects may be helpful in improving the elimination of foreign antigens, which have been implicated in the pathogenesis of Behçet's disease.57–59 Furthermore, it inhibits the proliferation of γδ T cells,60 which are increased and may have a pathological role in Behçet's disease.61 IFN also has immunosuppressive effects which could directly suppress vasculitis, as it does, for example, inhibit the adhesion of T cells to endothelial cells.62
Interferon alfa-2a may be superior to the standard immunosuppressive treatments especially for the treatment of severe attacks of panuveitis and retinal vasculitis with respect to its rapid action, potential for complete remissions with restoration of visual acuity and complete tapering of medication without relapse. A definitive evaluation of the efficacy of rhIFNα-2a in ocular Behçet's disease will require randomised controlled trials against azathioprine or cyclosporin A.
Acknowledgments
We thank Professor CA Müller, Tübingen, for the HLA analysis, Dr R Klein, Tübingen, for the detection of autoantibodies; U Rückwaldt and S Koch for their technical assistance; and Professor Graham Pawelec, Tübingen, for his critical review of the manuscript.
REFERENCES
- 1.Yazici H. Behçet's disease. In: Klippel JH, Dieppe PA, eds. Rheumatology. St Louis: Mosby 1998:7.26.1–2.
- 2.Behçet H. Über rezidivierende, aphthöse, durch ein Virus verursachte Geschwüre am Mund, am Auge und an den Genitalien. Dermatol Wschr 1937;36:1152–7. [Google Scholar]
- 3.Wechsler B. Lésions anatomo-pathologiques observeés au cours de la maladie de Behçet. Sém Hop Paris 1986;62:1337–40. [PubMed] [Google Scholar]
- 4.Shimizu T, Ehrlich G, Inaba G, et al. Behçet's disease. Sem Arthritis Rheum 1979;8:223–60. [DOI] [PubMed] [Google Scholar]
- 5.Main DMG, Chamberlain MA Clinical differentiation of oral ulceration in Behçet's disease. Br J Rheumatol 1992;31:767–70. [DOI] [PubMed] [Google Scholar]
- 6.BenEzra D, Cohen E. Treatment and visual prognosis in Behçet's disease. Br J Ophthalmol 1986;70:589–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochereau-Massin I, Wechsler B, Le-Hoang P, et al. Pronostic oculaire de la maladie de Behçet. J Fr Ophtalmol 1992;15:343–7. [PubMed] [Google Scholar]
- 8.Mishima S, Masuda K, Izawa Y, et al. Behçet's disease in Japan: ophthalmologic aspects. Trans Am Ophthalmol Soc 1979;57:225–79. [PMC free article] [PubMed] [Google Scholar]
- 9.Kötter I, Dürk H, Saal JG, et al. Therapy of Behçet's disease. Ger J Ophthalmol 1996;5:92–7. [PubMed] [Google Scholar]
- 10.Ando K, Fujino Y, Hijikata K, et al. Epidemiological features and visual prognosis of Behçet's disease. Jpn J Ophthalmol 1999;43:312–17. [DOI] [PubMed] [Google Scholar]
- 11.Yazici H, Pazarli H, Barnes CG, et al. A controlled trial of azathioprine in Behçet's syndrome. N Engl J Med 1990;322:281–5. [DOI] [PubMed] [Google Scholar]
- 12.Hamuryudan V, Özyazgan Y, Hizli N, et al. Azathioprine in Behçet's syndrome. Arthr Rheum 1997;40:769–74. [DOI] [PubMed] [Google Scholar]
- 13.BenEzra D, Cohen E, Chajek T, et al. Evaluation of conventional therapy versus cyclosporine A in Behçet's syndrome. Transplant Proc 1988;20:136–43. [PubMed] [Google Scholar]
- 14.Masuda K, Urayama A, Kogure M, et al. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behçet's disease. Lancet 1989;ii:1093–5. [DOI] [PubMed] [Google Scholar]
- 15.Nussenblatt RB, Palestine AG, Chan CC, et al. Effectiveness of cyclosporin therapy for Behçet's disease. Arthritis Rheum 1985;28:671–9. [DOI] [PubMed] [Google Scholar]
- 16.Özyazgan Y, Yurdakul S, Yazici H, et al. Low dose cyclosporin A versus pulsed cyclophosphamide in Behçet's syndrome: a single masked trial. Br J Ophthalmol 1992;76:241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Süllü Y, Öge I, Erkan D, et al. Cyclosporin-A therapy in severe uveitis of Behçet's disease. Acta Ophthalmol Scand 1998;76:96–9. [DOI] [PubMed] [Google Scholar]
- 18.Bang D. Treatment of Behçet's disease. Yonsei Med J 1997;38:401–10. [DOI] [PubMed] [Google Scholar]
- 19.Alpsoy E, Yilmaz E, Basaran E. Interferon therapy for Behçet's disease. J Am Acad Dermatol 1994;31:617–19. [DOI] [PubMed] [Google Scholar]
- 20.Hamuryudan V, Moral F, Yurdakul S, et al. Systemic interferon 2b treatment in Behçet's syndrome. J Rheumatol 1994;21:1098–100. [PubMed] [Google Scholar]
- 21.Zouboulis CC, Treudler R, Orfanos CE. Morbus Adamantiades-Behçet. Therapeutischer Einsatz von systemischem rekombinantem Interferon-2a. Hautarzt 1993;44:440–5. [PubMed] [Google Scholar]
- 22.Fierlbeck G, Rassner G. Rekombinantes Interferon-gamma bei Psoriasis arthropathica, progressiv-systemischer Sklerodermie und Morbus Behçet. Med Klin 1988;21:695–9. [PubMed] [Google Scholar]
- 23.Tsambaos D, Eichelberg D, Goos M. Behçet's syndrome: treatment with recombinant leukocyte alpha-interferon. Arch Dermatol Res 1986;278:335–6 [DOI] [PubMed] [Google Scholar]
- 24.Feron EJ, Rothova A, van Hagen PM, et al. Interferon-2b for refractory ocular Behçet's disease. Lancet 1994;343:1428. [DOI] [PubMed] [Google Scholar]
- 25.Durand JM, Kaplanski G, Telle H, et al. Beneficial effects of interferon-α2b in Behçet's disease. Arthritis Rheum 1993;36:1025. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler B, Bodaghi B, Le Thi Huong D, et al. Efficacy of interferon alfa-2a in severe and refractory uveitis associated with Behçet's disease. Ocular Immunol Inflamm 2000;8:293–301. [DOI] [PubMed] [Google Scholar]
- 27.Georgiou S, Monastirli A, Pasmatzi E, et al. Efficacy and safety of systemic recombinant interferon-alpha in Behçet's disease. J Intern Med 1998;243:367–72. [DOI] [PubMed] [Google Scholar]
- 28.Azizerli G, Sarica R, Köse A, et al. Interferon alfa-2a in the treatment of Behçet's disease. Dermatology 1996;192:239–41. [DOI] [PubMed] [Google Scholar]
- 29.Dündar S, Demiro H, Özcebe O, et al. Alpha interferon in Behçet's disease. Hematol Rev 1996;9:285–90. [Google Scholar]
- 30.Sánchez Román J, Aguilera Pulido MC, Castillo Palma MJ, et al. Utilizatión de interferón alfa 2r en el tratamiento de las uveítis autoimmunes (primarias o asociadas a enfermedad de Behçet). Rev Clin Esp 1996;196:293–8. [PubMed] [Google Scholar]
- 31.Kötter I, Eckstein AK, Stübiger N, et al. Treatment of ocular symptoms of Behçet's disease with interferon 2a: a pilot study. Br J Ophthalmol 1998;82:488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stübiger N, Kötter I, Deuter C, et al. Morbus Behçet: Uveitis—Therapie mit Interferon a2a—prospektive klinische (Pilot-) Studie an 33 Patienten. Klin Monatsbl Augenheilkd 2001;218:768–73. [DOI] [PubMed] [Google Scholar]
- 33.O'Duffy JD, Calamia K, Cohen S, et al. Interferon: treatment of Behçet's disease. J Rheumatol 1998;25:1938–44. [PubMed] [Google Scholar]
- 34.Demiroglu H, Özcebe OI, Barista I, et al. Interferon alfa-2b, colchicine, and benzathine penicillin versus colchicine and benzathin penicilline in Behçet's disease: a randomised trial. Lancet 2000;355:605–9. [DOI] [PubMed] [Google Scholar]
- 35.Eldem B. Behçet's disease and interferon: flaws in research integrity of randomised trial. Lancet 2000;356:1350. [DOI] [PubMed] [Google Scholar]
- 36.Horton R. Retraction: interferon alfa-2b . . . in Behçet's disease. Lancet 2000;356:1292. [DOI] [PubMed] [Google Scholar]
- 37.Demiroglu H. Behçet's disease and interferon: flaws in research and integrity of randomized trial. Lancet 2000;356:1341–52. [DOI] [PubMed] [Google Scholar]
- 38.Kötter I, Aepinus C, Graepler F, et al. HHV8 associated Kaposi's sarcoma during triple immunosuppressive treatment with cyclosporin A, azathioprine, and prednisolone for ocular Behçet's Disease and complete remission of both disorders with interferon A. Ann Rheum Dis 2001;60:83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. Lancet 1990;335:1078–80. [PubMed] [Google Scholar]
- 40.BenEzra D, Forrester JV, Nussenblatt RB, et al. Uveitis scoring system. Berlin: Springer Verlag, 1991.
- 41.Rigby AS, Chamberlain MA, Bhakta B. Behçet's disease. In: Classification and assessment of rheumatic diseases: Part I Bailliere's clinical rheumatology. 1995;9:375–95. [DOI] [PubMed] [Google Scholar]
- 42.Kötter I, Dürk H, Eckstein A, et al. Erosive arthritis and posterior uveitis in Behçet's disease: treatment with interferon α and interferon γ. Clin Exp Rheumatol 1996;14:313–15. [PubMed] [Google Scholar]
- 43.Fritzsch J, Krug J, Heberling HJ. Interferontherapie und Autoimmunität. Med Klin 1997;5:265–72. [DOI] [PubMed] [Google Scholar]
- 44.Rönnblom LE, Alm GV, Öberg KE. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med 1991;115:178–83. [DOI] [PubMed] [Google Scholar]
- 45.Fattovich G, Betterle C, Brollo L, et al. Autoantibodies during alpha-interferon threapy for chronic hepatitis B. Br J Med Virol 1991;34: 132–5. [DOI] [PubMed] [Google Scholar]
- 46.Wandl UB, Nagel-Hiemke M, May D, et al. Lupus-like autoimmune disease induced by interferon therapy for myeloproliferative disorders. Clin Immunol Immunopathol 1992;65:70–4. [DOI] [PubMed] [Google Scholar]
- 47.Prezati D, La Rosa L, Covini G, et al. Autoimmunity and thyroid function in patients with chronic active hepatitis treated with recombinant interferon alpha-2a. Eur J Endocrinol 1995;132:587–93. [DOI] [PubMed] [Google Scholar]
- 48.Bennett R. The concurrence of lupus and fibromyalgia: implications for diagnosis and management. Lupus 1997;6:494–9. [DOI] [PubMed] [Google Scholar]
- 49.Quesada JR, Gutterman JU. Psoriasis and alpha-interferon. Lancet 1986;i:1466–8. [DOI] [PubMed] [Google Scholar]
- 50.Guyer DR, Tiedemann J, Yannuzzi LA, et al. Interferon-associated retinopathy. Arch Ophthalmol 1993;111:350–6. [DOI] [PubMed] [Google Scholar]
- 51.Kawano T, Shigehira M, Uto H, et al. Retinal complications during interferon therapy for chronic hepatitis C. Am J Gastroenterol 1996:309–13. [PubMed]
- 52.Purvin VA. Anterior ischemic optic neuropathy secondary to interferon alfa. Arch Ophthalmol 1995;113:1041–4. [DOI] [PubMed] [Google Scholar]
- 53.Pateron D, Fain O, Sehonnu J, et al. Severe necrotizing vasculitis in a patient with hepatitis C virus infection treated by interferon. Clin Exp Rheumatol 1996;14:79–81. [PubMed] [Google Scholar]
- 54.Segawa F, Shimizu Y, Saito E, et al. Behçet's disease induced by interferon therapy for chronic myelogenous leukemia. J Rheumatol 1995;22:1183–4. [PubMed] [Google Scholar]
- 55.Budak-Alpdogan T, DemirHay Z, Alpdogan Ö, et al. Behçet's disease in patients with chronic myelogenous leukemia: possible role of interferon-allpha treatment in the occurrence of Behαet's symptoms. Ann Hematol 1997;74:45–8. [DOI] [PubMed] [Google Scholar]
- 56.Belardelli F, Gresser I. The neglected role of type I interferon in the T-cell response: implications for its clinical use. Immunol Today 1996;17:369–72. [DOI] [PubMed] [Google Scholar]
- 57.Young C, Lehner T, Barnes CG. CD4 and CD8 cell responses to herpes simplex virus in Behçet's disease. Clin Exp Immunol 1988;73:6–10. [PMC free article] [PubMed] [Google Scholar]
- 58.Kiraz S, Ertenli I, Benekli M, et al. Parvovirus B19 infection in Behçet's disease. Clin Exp Rheumatol 1996;14:71–3. [PubMed] [Google Scholar]
- 59.Lehner T, Fortune F, Studd M. T cell immunomodulation in Behçet's disease and consideration of microbial etiology involving herpes simplex virus, heat shock proteins and streptococcal antigens. In: O'Duffy JD, Kokmen E, eds. Behçet's disease. New York: Marcel Dekker, 1995:463–73.
- 60.Metzger R, Heckl-Österreicher B, Nerl C, et al. Immunological studies of T cells in a case of large granular lymphocyte (LGL) leukemia: leukemic + T cells are resistant to growth stimulation in vitro but respond to interferon-α treatment in vivo. Leukemia Res 1992;16:1087–95. [DOI] [PubMed] [Google Scholar]
- 61.Hasan A, Fortune F, Wilson A, et al. Role of T cells in pathogenesis and diagnosis of Behçet's disease. Lancet 1996;347:789–94. [DOI] [PubMed] [Google Scholar]
- 62.Eguchi K, Kawakami A, Nakashima M, et al. Interferon-alpha and dexamethasone inhibit adhesion of T cells to endothelial cells and synovial cells. Clin Exp Immunol 1992;88:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]