Abstract
Aims: To investigate the staining pattern of neurotrophin-3 (NT3), neurotrophin-4 (NT4), and brain derived neurotrophic factor (BDNF) as well as glial fibrillary acid protein (GFAP) and CD68 in lasered human retina.
Methods: Retinal laser photocoagulation was performed on four patients (two males, two females) with choroidal malignant melanoma 1–6 days before enucleation. Three other enucleated eyes with malignant melanoma and three normal cadaveric donor eyes were used as controls. Immunohistochemistry was performed to investigate the pattern of staining of NT3, NT4, BDNF, GFAP, and CD68 in 7 mm sections of fixed specimens.
Results: Expression of NT4 was detected in the inner and outer nuclear layers of all the retinal sections examined but no NT3 and BDNF staining was seen. NT4 staining was found to be less intense in lasered and melanoma controls compared to normal cadaveric donor retinas. There was an upregulation of GFAP expression in both lasered and control eyes with melanoma in comparison with normal controls. CD68 staining was only observed in retinal pigment epithelium and choroid of lasered eyes.
Conclusion: NT4 is expressed in inner and outer nuclear layers of normal human retina and its expression is downregulated following laser photocoagulation. This occurs in parallel with an increased expression of GFAP suggesting that reactive changes in Muller cells may be responsible for reduced NT4 staining. Expression of CD68 at the site of laser injury is consistent with a wound healing process as a response to local damage.
Keywords: laser photocoagulation, neurotrophins, Muller cells, retinal pigment epithelium, GFAP
L aser photocoagulation is an established treatment for a number of retinal neovascular diseases; however, the mechanism of its action is not yet fully understood. Retinal photocoagulation causes outer retinal necrosis, hyperplasia of the retinal pigment epithelium (RPE), and glial cell proliferation and migration, eventually leading to localised fibrous scar formation.1–3 Cellular activation may be reflected in changes of expression of various markers such as glial fibrillary acidic protein (GFAP), which is highly expressed by astrocytes but minimally expressed by resting Muller cells, which increase their expression of GFAP when activated.4 Similarly, RPE cells, which do not normally stain for CD68 in vivo, express this molecule under culture conditions as evidence of activation.5 Upregulation of cell marker expression has become an important marker of injury in central nervous system tissues including the retina,4,6 where GFAP upregulation in Muller cells occurs rapidly after retinal detachment7 as well as following laser photocoagulation.4,8
Neurotrophins (NT) are trophic and mitogenic proteins that have a role in the development, differentiation, connectivity, and survival of neurons in the central and peripheral nervous system, including the retina. Brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), and neurotrophin-4 (NT4) have been demonstrated to promote retinal ganglion cell survival after injury and BDNF is thought to aid in the recovery of the retina after reattachment.9–14 NT4 (also called NT5) is the most recently discovered NT in mammals and its biological role is not fully understood. All NT knockout mice have proved lethal during early postnatal development apart from NT4 deficient mice that only show minor cellular deficits and develop normally to adulthood.15 NT4 knockout mice have recently been reported to require NT3 in early postnatal development and NT4 later in mature animals for survival of the sensory neurons.16 The biological activities of neurotrophins are mediated by two classes of cell surface receptors: the neurotrophin receptor p75, which binds all neurotrophins with similar affinity, and the trk family of receptor tyrosine kinases. Each of the three trk receptors has preferential ligands (NGF for Trk A, BDNF and NT4 for Trk B, and NT3 for Trk C).17–19
The cellular and molecular events induced by retinal photocoagulation and the subsequent wound healing response are complex and as yet incompletely characterised processes. Experimental models suggest that these events are regulated by a variety of growth factors,20 but neurotrophins so far have not been implicated. The purpose of this study was to analyse alterations in protein expression of the neurotrophins, BDNF, NT3 and NT4 in lasered human retina. The expression of GFAP and the macrophage marker CD68 in the laser induced retinal lesions were also assessed to determine the relation of neurotrophin expression to other markers of the retinal response to laser injury.
PATIENTS AND METHODS
Specimens
Laser photocoagulation was performed on the retinas of four patients (two males and two females, median age 70 years) before undergoing enucleation for malignant melanoma of the choroid (Table 1). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and approved by Moorfields Eye Hospital research ethics committee.
Table 1.
Features of laser treated melanoma eyes
| Specimen | Age | Eye | Tumour location | Location of laser burns | Laser spot energy |
| 1 | 70 | left | macular | nasal | 400–700 mW |
| 2 | 82 | left | inferior | superior | 410–650 mW |
| 3 | 70 | right | superior | inferior | 30–60 mW |
| 4 | 47 | right | inferior | superior | 30–60 mW |
Patients with pre-existing retinal disease or clinically significant retinal detachment associated with the choroidal melanoma were excluded from the study. All four patients received three rows of 10–15 creamy white argon green laser burns away from the choroidal melanoma. Laser photocoagulation was performed 1 day before enucleation in one patient, 2 days before enucleation in one patient, and 6 days before enucleation in two patients. Laser spots were 500 μm and of 0.1 second duration. Other laser treatment details are summarised in Table 1. Eyes were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) immediately after enucleation.
Immunohistochemical staining
Retinal tissue containing the laser foci were paraffin embedded and sections of 7 μm thickness prepared for immunohistochemistry. Retinal sections from three non-lasered eyes with malignant choroidal melanoma and three normal eyes from corneal donors with normal retina were used as controls. Slides were immersed in pepsin solution (0.4 g in 100 ml of 0.1N HCl) at 37°C for 15 minutes, washed twice in TRIS buffered saline (TBS) and placed in 0.5% blocking reagent (Roche Diagnostics, Germany) in TBS for 20 minutes. Sections were incubated overnight at room temperature with a panel of primary monoclonal antibodies diluted in 0.5% blocking reagent in TBS as follows: anti-BDNF (MAB248, 10 μg/ml, R&D Systems, UK); anti-NT3 (MAB267, 5 μg/ml, R&D Systems, UK); anti-NT4 (MAB268, 5 μg/ml, R&D Systems, UK); anti-GFAP (Clone 6F2, 10 μg/ml Dako, Denmark); and anti-CD68 (Clone PG-M1, 10 μg/ml, Dako, Denmark). Mouse IgG isotypes matching those of the test antibodies (Sigma, UK) were used as negative controls in the assay. Following incubation with primary antibody, specimens were washed and further incubated for 30 minutes with 50 μl of biotin labelled rabbit anti-mouse antibody (Dako, UK). After washing in TBS, slides were then covered with 50 μl streptavidin-AP complex (Dako, Denmark) and incubated for 45 minutes, washed twice with TBS, and further incubated for 30 minutes with 50 μl of alkaline phosphate substrate (Vector Red Vector Laboratories, UK). Slides were counterstained with Mayer's haematoxylin before microscopy analysis. Selected non-immunolabelled sections were also stained with haematoxylin and eosin to permit evaluation of the overall morphology of the sections. Slides were analysed by three different observers under light microscopy and there was complete agreement in their findings during slide analysis.
RESULTS
Neurotrophin expression
No immunostaining for NT-3 or BDNF was detected in any of the retinas from eyes that had undergone laser treatment or in controls (Table 2).
Table 2.
Immunohistochemical staining of lasered and control retinas
| Specimen number | Origin of specimen | Laser before enucleation (days) | NT4 | GFAP | CD68 |
| 1 | Melanoma | 1 | +* | +++‡ | + |
| 2 | Melanoma | 2 | +* | +++‡ | + |
| 3 | Melanoma | 6 | +* | +++‡ | ++ |
| 4 | Melanoma | 6 | +* | +++‡ | ++ |
| 5 | Melanoma | NA | +† | +++‡ | − |
| 6 | Melanoma | NA | +† | +++‡ | − |
| 7 | Melanoma | NA | − | +++‡ | − |
| 8 | Cadaveric donor | NA | ++† | +§ | − |
| 9 | Cadaveric donor | NA | ++† | +§ | − |
| 10 | Cadaveric donor | NA | ++† | +§ | − |
Intensity of staining: +++ = strong, ++ = moderate, + = mild, − = negative. *Staining reduced in outer nuclear layer at the site of the laser burn, †staining of inner and outer nuclear layers of the retina, ‡staining throughout the whole retina, §scattered staining of cells predominantly in the nerve fibre layer.
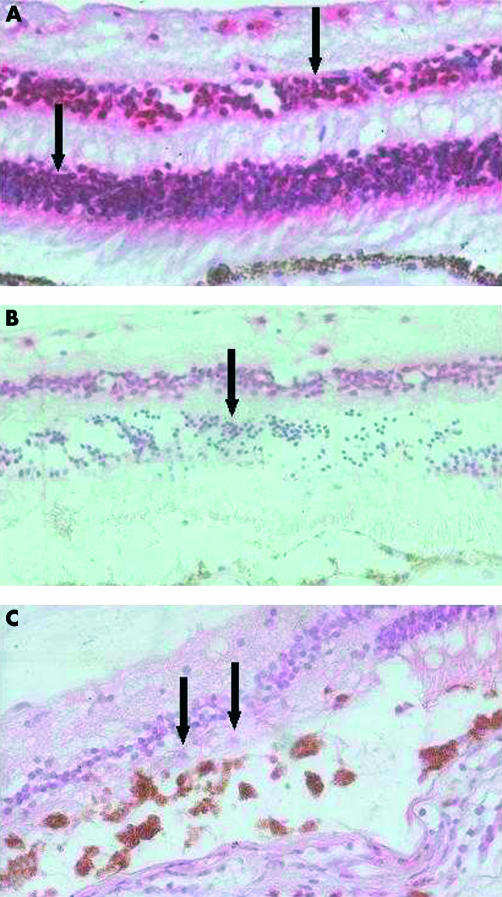
Immunostaining for NT4 however was observed in both the inner and outer nuclear and ganglion cell layers in two out of three retinas of melanoma patients and all normal controls (Fig 1A, Table 2). However, in the melanoma eye which underwent laser photocoagulation 1 day before enucleation, there was a marked decrease in the immunostaining for this neurotrophin in the outer nuclear cell layer at the site of the laser burn (Table 2, Fig 1B). In eyes enucleated 6 days after laser treatment, the outer nuclear layer was atrophic at the laser site, but occasional cells still present, were negative for this molecule (Fig 1C).
Figure 1.
Pattern of NT4 staining in normal and laser treated retinas. Haematoxylin and eosin and anti-NT4 stain. (A) Normal retina showing intense NT4 staining in inner and outer nuclear layers (arrows), 50× magnification. (B) One day post-laser showing reduced NT4 staining in ONL (arrow). This reduced staining is confined to the extent of laser injury, 50× magnification. (C) Six days post-laser showing atrophic ONL at the site of laser with only a few cells seen and reduced NT4 staining (arrows), 100× magnification.
GFAP and CD68 expression
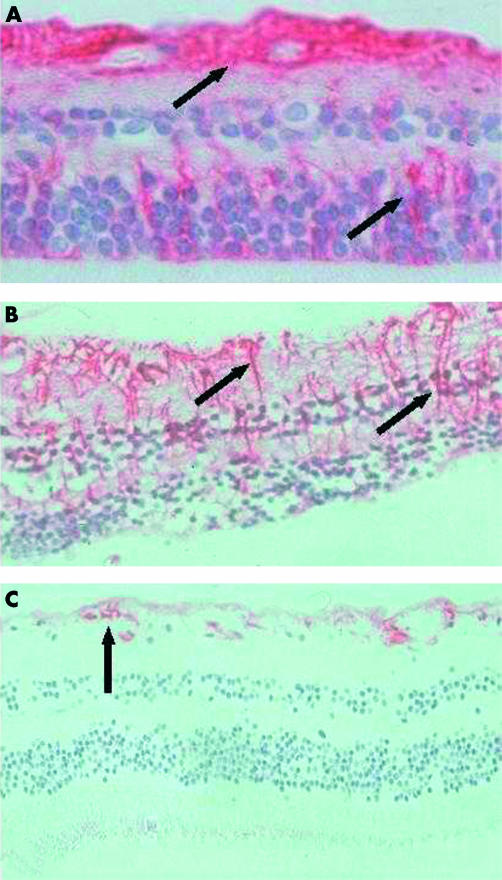
Intense staining for GFAP was observed throughout all the retinal layers in all melanoma eyes (2A) and no change in this expression was found in lasered eyes (Fig 2B). In contrast, retina from normal controls showed minimal staining for GFAP, which was limited to the ILM, ganglion cell, and nerve fibre layers (Fig 2C).
Figure 2.
Pattern of GFAP staining in malignant melanoma, laser treated, and normal retinas. Haematoxylin and eosin and anti-GFAP stain. (A) GFAP staining throughout all layers of retina in malignant melanoma, 50× magnification. (B) GFAP staining throughout all layers of the lasered retina (1 day post-laser), 50× magnification. (C) GFAP staining of the inner limiting membrane, ganglion cell, and nerve fibre layer in normal retina, 50× magnification.
Staining for CD68 was observed on laser damaged retinal pigment epithelial cells and on underlying choroidal inflammatory and pigmented cells. Cells positive for this marker were not observed in any of the control specimens (Fig 3A). In the eye enucleated 1 day after laser treatment there was a marked localised accumulation of polymorphonuclear leucocytes, mononuclear cells, and pigmented choroidal cells at the laser site (Fig 3B). In addition, a moderate number of scattered cells staining for CD68 were observed in the choroid, and mild RPE cells staining for CD68 was seen (Fig 3B). In the eye enucleated 2 days after laser, a more intense infiltration of CD68 positive cells, some of which formed an incomplete perivascular cuff, was observed, and RPE cells displayed a more intense CD68 staining than on day 1 (Fig 3C). In contrast, intense staining for CD68 was observed on mononuclear and choroidal pigmented cells as well as RPE cells 6 days after laser treatment (Fig 3D).
Figure 3.
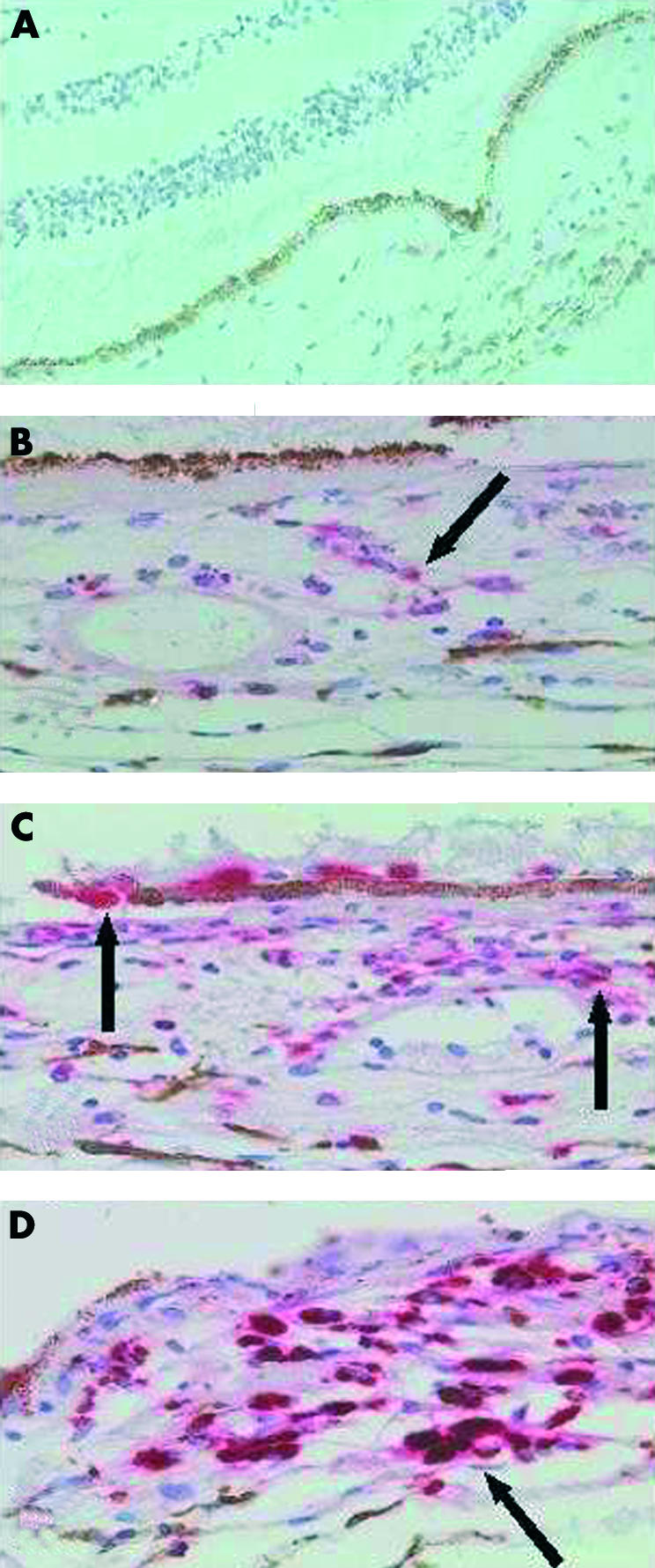
Pattern of CD68 staining in normal and laser treated retinas. Haematoxylin and eosin and anti-CD68 antibody. (A) Normal retina with no CD68 staining in the choroid, 50× magnification. (B) CD68 staining in the choroid 1 day post-laser, 100× magnification. (C) CD68 staining 2 days post-laser. Note CD68 positive cells associated with RPE and perivascular cuff, 100× magnification. (D) CD68 staining 6 days post-laser showing more intense staining compared to days 1 and 2, 100× magnification.
DISCUSSION
Our immunohistochemical findings showed that normal human retina expresses NT4 at the level of outer nuclear layer (ONL), inner nuclear layer (INL) and ganglion cell layer (GCL), and that NT4 expression was downregulated at the site of laser burns when examined at 1, 2, and 6 days post-laser. The degree of downregulation was more marked in the eyes enucleated 6 days after laser treatment and less on those enucleated after 1–2 days. These observations suggest that NT4 downregulation may be either secondary to local cell damage and loss at ONL level or it may reflect a true reduction in NT4 expression in cells normally producing these proteins.
Reduced intensity of NT4 staining was also noted in two of the melanoma patients compared with normal cadaveric retinas and NT4 staining was absent in one case (Table 2). This suggests reduced NT4 production, increased NT4 degradation, or a combination of both. It is also possible that the observed GFAP upregulation, indicating Muller cell reactivity, in melanoma and laser treated retinas is associated with the reduced NT4 staining. In vitro studies suggest that there is an increased sequestration of BDNF and NT4 at the site of reactive gliosis in the central nervous system.21,22
NT4 can reduce cell death by binding to and activating its receptor TrkB.14 BDNF and NT4 bind to the same receptor and can produce similar neurotrophic effect on RGC in vitro,23 suggesting that there may be overlapping biological effects mediated by NT4 and BDNF acting via TrkB receptor. In vivo intraocular injection of BDNF rescues significant numbers of retinal ganglion cells that would have otherwise died following section of the adult rat optic nerve.11 It has been proposed that BDNF may aid in the recovery of the retina after reattachment by maintaining the surviving photoreceptor cells, by reducing the gliotic effects in Muller cells, and perhaps by promoting outer segment regeneration.13 The loss of support from NT4 (and possibly other neurotrophins) could potentially be a factor in degeneration and apoptotic cell death found in the retina in various pathologies.24
The immunohistochemical staining used in this study did not detect BDNF or NT3 in any of the retinas examined. These findings do not correlate with previous reports in a variety of vertebrate retinas.9,25–27 It may be possible that the antibodies used in this study did not recognise the epitopes expressed in PFA fixed tissues, or that the method used was not sensitive enough. Strong expression of GFAP was observed in control melanoma eyes as well as laser treated eyes, but minimal expression of these molecules was seen in normal controls. Increased levels of this protein have been reported in the Muller cells following laser exposure in rabbit eyes,4,8 where GFAP immunoreactivity in Muller cells was observed as far as 4–5 mm from the laser foci which was much larger than the RPE disrupted area.8 Our findings suggest that retinal GFAP expression is increased in the presence of melanoma and laser treatment did not modify this. It is notable that an increased immunoreactivity for GFAP has also been observed in retinal explants when exposed to choroidal melanoma cells ex vivo.28 GFAP may be maximally expressed in melanoma eyes and therefore it may not be possible to detect a further increase following laser treatment.
The expression of CD68 was only observed at the site of the laser treated retinas and not in the normal or melanoma affected eyes. Immunoreactive cells were localised in the laser damaged RPE as well as in the underlying choroid. CD68 is a cell surface marker for macrophages and has previously been reported to be expressed by cultured RPE cell.5,29 Macrophages and leucocyte infiltrates characterise inflammatory responses and their presence in the choroid in foci of laser disruption may represent a secondary wound repair response. Previous studies have shown that the origin of the mononuclear leucocytes that accumulate at the site of laser burns is the systemic circulation.30,31
Our study used immunohistochemical analysis and our findings agree with those previously reported,32 where RPE cells in specimens obtained from eyes with various ocular diseases including intraocular melanoma did not react with antibody to CD68. This observation suggests that laser damaged RPE cells and those cultured5,27 (“normal”) may have undergone activation, not seen in normal and diseased in vivo cells.
CONCLUSION
Functional changes in RPE cells, demonstrated by changes in CD68 expression and downregulation of the NT4 in the ONL may be responsible for some of the changes seen in the retinal function after laser. Alterations in the expression of other neurotrophins and their receptors may also have a role in the response of the retina to injuries of various types. Further studies are needed to investigate the role of neurotrophins in the pathobiology of wound repair in the retina.
REFERENCES
- 1.Van der Zypen E, Fankhauser F, Raess K, et al. Morphologic findings in the rabbit retina following irradiation with the free-running neodymium-YAG laser. Disruption of Bruch's membrane and its effect on the scarring process in the retina and choroids. Arch Ophthalmol 1986;104:1070–7. [DOI] [PubMed] [Google Scholar]
- 2.Marshall J, Bird A. A comparative histopathological study of argon and krypton laser irradiations of the human retina. Br J Ophthalmol 1979; 63:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobe T, Takahashi K, Kishimoto N, et al. Effects of interferon-beta on repair of the retinal pigment epithelium after laser photocoagulation. Nippon Ganka Gakkai Zasshi Acta Soc Ophthalmol Jap 1995; 99:792–805. [PubMed] [Google Scholar]
- 4.Humphrey MF, Chu Y, Mann K, et al. Retinal GFAP and bFGF expression after multiple argon laser photocoagulation injuries assessed by both immunoreactivity and mRNA levels. Exp Eye Res 1997; 64:361–9. [DOI] [PubMed] [Google Scholar]
- 5.Limb GA, Cole CJ, Earley O, et al. Expression of hematopoietic cell markers by retinal pigment epithelial cells. Curr Eye Res 1997;16:985–91. [DOI] [PubMed] [Google Scholar]
- 6.Lewis GP, Erickson PA, Guerin CJ, et al. Changes in the expression of specific Muller cell proteins during long-term retinal detachment. Exp Eye Res 1989; 49:93–111. [DOI] [PubMed] [Google Scholar]
- 7.Erickson PA, Fisher SK, Guerin CJ, et al. Glial fibrillary acidic protein increases in Muller cells after retinal detachment. Exp Eye Res 1987;44:37–48. [DOI] [PubMed] [Google Scholar]
- 8.Humphrey MF, Constable IJ, Chu Y, et al. A quantitative study of the lateral spread of Muller cell responses to retinal lesions in the rabbit. J Comp Neurol 1993;334:545–58. [DOI] [PubMed] [Google Scholar]
- 9.Das I, Hempstead BL, MacLeish PR, et al. Immunohistochemical analysis of the neurotrophins BDNF and NT-3 and their receptors trk B, trk C, and p75 in the developing chick retina. Vis Neurosci 1997;14:835–42. [DOI] [PubMed] [Google Scholar]
- 10.De la Rosa EJ, Arribas A, Frade JM, et al. Role of neurotrophins in the control of neural development: neurotrophin-3 promotes both neuron differentiation and survival of cultured chick retinal cells. Neuroscience 1994;58:347–52. [DOI] [PubMed] [Google Scholar]
- 11.Mansour-Robaey S, Clarke DB, Wang YC, et al. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA 1994;91:1632–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Polo A, Aigner LJ, Dunn RJ, et al. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci USA 1998;95:3978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis GP, Linberg KA, Geller SF, et al. Effects of the neurotrophin brain-derived neurotrophic factor in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci 1999; 40:1530–44. [PubMed] [Google Scholar]
- 14.Cui Q, Harvey AR. NT-4/5 reduces naturally occurring retinal ganglion cell death in neonatal rats. Neuroreport 1994;5:1882–4. [DOI] [PubMed] [Google Scholar]
- 15.Ibanez CF. Neurotrophin-4: the odd one out in the neurotrophin family.Neurochem Res 1996; 21:787–93. [DOI] [PubMed] [Google Scholar]
- 16.Stucky C, Shin J, Lewin G.Neurotrophin-4. A survival factor for adult sensory neurons. Curr Biol 2002;12:1401–4. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan DR, Hempstead BL, Martin-Zanca D, et al. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science 1991;252:554–8. [DOI] [PubMed] [Google Scholar]
- 18.Soppet D, Escandon E, Maragos J, et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 1991;65:895–903. [DOI] [PubMed] [Google Scholar]
- 19.Lamballe F, Klein R, Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell 1991;66:967–79. [DOI] [PubMed] [Google Scholar]
- 20.Xiao M, McLeod D, Cranley J, et al. Growth factor staining patterns in the pig retina following retinal laser photocoagulation. Br J Ophthalmol 1999; 83:728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alderson RF, Curtis R, Alterman AL, et al. Truncated TrkB mediates the endocytosis and release of BDNF and neurotrophin-4/5 by rat astrocytes and schwann cells in vitro. Brain Res 2000;871:210–22. [DOI] [PubMed] [Google Scholar]
- 22.Fryer RH, Kaplan DR, Kromer LF. Truncated trkB receptors on nonneuronal cells inhibit BDNF-induced neurite outgrowth in vitro. Exp Neurol 1997;148:616–27. [DOI] [PubMed] [Google Scholar]
- 23.Cohen A, Bray GM, Aguayo AJ. Neurotrophin-4/5 (NT-4/5) increases adult rat retinal ganglion cell survival and neurite outgrowth in vitro. J Neurobiol 1994; 25:953–9. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res 2001;86:1–12. [DOI] [PubMed] [Google Scholar]
- 25.Bennett JL, Zeiler SR, Jones KR. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Invest Ophthalmol Vis Sci 1999;40:2996–3005. [PubMed] [Google Scholar]
- 26.Hallbook F, Backstrom A, Kullander K, et al. Expression of neurotrophins and trk receptors in the avian retina. J Comp Neurol 1996;364:664–76. [DOI] [PubMed] [Google Scholar]
- 27.Cellerino A, Kohler K. Brain-derived neurotrophic factor/neurotrophin-4 receptor TrkB is localized on ganglion cells and dopaminergic amacrine cells in the vertebrate retina. J Comp Neurol 1997;386:149–60. [PubMed] [Google Scholar]
- 28.Enzmann V, Germer A, Francke M, et al. Alterations of sensory retinal explants exposed to choroidal melanoma cells ex vivo. Graefes Arch Clin Exp Ophthalmol 2000;238:985–92. [DOI] [PubMed] [Google Scholar]
- 29.Elner SG, Elner VM, Nielsen JC, et al. CD68 antigen expression by human retinal pigment epithelial cells. Exp Eye Res 1992;55:21–8. [DOI] [PubMed] [Google Scholar]
- 30.Martini B, Ryan SJ. Argon laser lesions of the retina; occurrence and origin of macrophages. Eur J Ophthalmol 1992; 2:51–7. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa Y, Momoeda S, Yoshitomi F. Origin of macrophage in photocoagulated rabbit retina. Jap J Ophthalmol 1983;27:138–48. [PubMed] [Google Scholar]
- 32.Nicolai U, Eckardt C. The occurrence of macrophages in the retina and periretinal tissues in ocular diseases. German J Ophthalmol 1993;2:195–201. [PubMed] [Google Scholar]