Abstract
Aims: To assess the adequacy of current decontamination methods for the Goldmann tonometer in the context of variant Creutzfeldt-Jakob disease (vCJD).
Methods: Reusable Goldmann tonometer prisms were used to perform applanation tonometry on different groups of patients. Following tonometry, retained materials were collected from the tonometer prism head and examined using cytological methods. The used tonometers were subjected to a series of conditions to evaluate their effect on the residual cell numbers found on the tonometer heads. These included wiping alone and wiping or washing followed by disinfection of the tonometer prism. The effect on cell counts of drying the prism overnight was studied, as well as drying overnight and then wiping and disinfecting. All disinfections were performed with sodium hypochlorite (0.05% w/v).
Results: The cytology specimens of 69 patients were studied. Patients using eye drops regularly desquamated significantly more corneal epithelial cells with Goldmann tonometry than patients not using regular eye drops. The mean number of cells was 156 (range 0–470) for patients using eye drops and 14 (4–57) for patients not using eye drops (p = 0.004). Wiping or washing the tonometer head reduced the cell number significantly but neither method completely eliminated cells. The two methods were not significantly different (p=0.3). Drying left a large number of cells (23–320 cells).
Conclusions: Retained corneal epithelial cells, following the standard decontamination routine of tonometer prisms, may represent potential prion infectivity. Manual cleaning was the most important step in reducing epithelial cell retention.
Keywords: applanation tonometry, cornea, Creutzfeldt-Jakob disease, cytology, decontamination
Creutzfeldt-Jakob disease (CJD) is the commonest human transmissible spongiform encephalopathy (TSE). Since the advent of variant CJD (vCJD) in the United Kingdom, the level of concern about the decontamination of reused instruments has risen, since the number of individuals incubating vCJD is unknown (estimates range from a few hundred to many thousands of potential cases). Much is still not known about CJD, vCJD, and their transmission. The cornea is considered a potential site of transmission as four cases of CJD transmission via cadaveric corneal transplants have been reported (one definite, one probable, and two possible cases).1,2 Also, experimental transmission of CJD with infected cornea has been reported from humans to mice (intracerebral injection)3 and between guinea pigs (intraocular injection).4
Applanation tonometry has been proposed as a risk for CJD and now vCJD.1,5–8 Patients who undergo the procedure regularly, such as glaucoma patients, may be at increased risk of CJD and vCJD.6 Davanipour et al in their case-control study reported an increased odds of ocular tonometry testing in cases when compared to controls.5 The pooled European surveillance data, however, did not find an association between ophthalmological tests and CJD.9 Davanipour et al did not distinguish between contact and non-contact tonometry; this potentially reduced their positive finding. The pooled European data did not subdivide possible ophthalmological tests and their finding should be treated with circumspection. Eye medications, used chronically, may lead to corneal epithelial toxicity and possibly a higher likelihood of epithelial desquamation with tonometry. It is proposed that desquamated cells may contain the infective agent.
Sporadic CJD is rare and has an annual incidence of one per million.1 By February 2003 there were 131 definite or probable vCJD cases (dead and alive) in the United Kingdom, six in France, and one in each of the Republic of Ireland, Italy, United States, and Canada. Sporadic CJD is a disease of older people and it frequently presents as a dementing illness. About 10% of sporadic CJD cases comprise the Heidenhain variant with predominantly visual symptoms.1 Patients undergoing tonometry therefore could be carriers of latent CJD or vCJD and potential sources of iatrogenic spread.
To date there has been no study that has tried to assess CJD risk with tonometry. This hospital based study had been designed to assess the amount of corneal epithelial desquamation with Goldmann tonometry and to assess the effectiveness of routine cleaning and disinfection regimes with sodium hypochlorite 0.05% w/v, 500 ppm (parts per million).
PATIENTS AND METHODS
Patients were recruited from the Princess Alexandra Eye Pavilion ophthalmology outpatient clinics. Applanation tonometry was performed on patients in the usual way on both eyes. One of the authors (RL) and a senior ophthalmic nurse performed all of the tonometry. Reusable tonometer prisms available in the clinic were used. We studied tonometer prisms in current use as we were interested in how much corneal desquamation was occurring with these; we wanted to study “real life” practice within a large NHS ophthalmic unit. Tonometer tips were cleaned manually under running water thoroughly before disinfection and reuse. Particular attention was paid to the washing and manual cleaning to reduce the risk of contamination between successive experiments. Initially, patients using eye drops were compared to patients not using eye drops (10 patients in each group). These patients were recruited from the cataract assessment clinic and the glaucoma clinics. A comparison of the degree of corneal epithelial cell desquamation onto the tonometer prism head was made between these two groups.
For the next part of the study only glaucoma patients using eye drops were recruited. The rationale was that for the assessment of adequacy of cleaning and decontamination, patients with higher desquamation rates should be used. The tonometer prism head was subjected to various procedures.
Wipe tonometer head with tissue
Wipe with tissue and then place in sodium hypochlorite solution for 10 minutes
Dry for 24 hours
Dry for 24 hours, then wipe with tissue and place in sodium hypochlorite solution for 10 minutes
Wash tonometer head under running water with tissue and then place in sodium hypochlorite for 10 minutes.
All the sodium hypochlorite solution used in this study was 0.05% w/v. Following each process, the number of residual desquamated cells was quantified. Adhesive tape was used to remove material from the tonometer head surface and then the tape was applied to a slide. The slide was marked, using a diamond marker, to indicate the location of the tonometer head impressions. The slides were then sent to a single cytology laboratory for processing. Gloves were worn for all tonometer and slide handling. In the cytology laboratory, the specimens were fixed with heat; adhesive tape was removed using an overnight soak in hot xyline and then further rinsed with 100% alcohol before staining with haematoxylin and eosin.
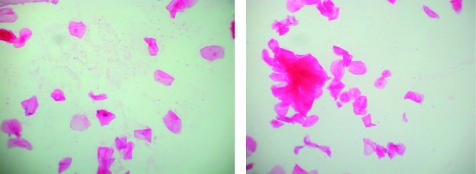
A baseline slide was made to check for contamination rates from handling by examiners and laboratory staff. This slide was handled in the same way as the specimen slides except the tonometer was not used on a patient. One examiner (KMK) scored all the slides, which were later reviewed (by JWI). The examiner was masked to the strategy used for each slide. The number of cells seen in the marked area of the slide were counted. Where there were clumps of cells (Fig 1) an estimate was made of the number of cells within the clump.
Figure 1.
Two examples of retained cellular material on tonometer prism surfaces before manual cleaning. Nucleated cells and groups of dumped cells are present.
The Kruskal-Wallis non-parametric one way analysis of variance was performed to assess the relation between all the groups with respect to cells counted on the tonometer prism, age of the patient, and number of eye drops used by the patient. Specific associations between groups were assessed with the Mann-Whitney U test for non-parametric data. Bonferroni adjustments were also performed for multiple Mann-Whitney U comparisons. The statistical analysis was performed using spss for Windows (Version 7.0, CA, USA). A p value of <0.05 has been taken to represent statistical significance.
RESULTS
The baseline slide had three cells. Tonometry specimens from a total of 69 patients were included in the study. The groups were compared using a Kruskal-Wallis non-parametric one way ANOVA. The χ2 test for cell count was 33.5 with 6 degrees of freedom and an associated probability of p<0.001. Following the significant Kruskal-Wallis result for cell count, a number of paired comparisons between groups were carried out using the Mann-Whitney U test. As Bonferroni correction did not alter the decision on significance, the Mann-Whitney U test results without Bonferroni correction are presented.
The main results are shown in Table 1. Patients with “glaucoma on eye drops,” group 2, lost more cells with Goldmann tonometry when compared to “cataract patients” not using eye drops, group 1 (z = 2.88, p = 0.004). The ranges of cells were 4–57 from patients not using eye drops and 0–470 for people using regular eye drops. Tonometer heads that had been wiped once, group 3, had fewer cells than those that had not been wiped, group 2. This difference was significant (p = 0.004). The difference in number of cells found between wiping once, group 3, and wiping followed by sodium hypochlorite, group 4, was not significantly different (z=1.07, p = 0.3). Neither wiping nor wiping followed by immersion in sodium hypochlorite for 10 minutes removed cells completely from the tonometer surface (Table 1).
Table 1.
Retained corneal epithelial cell count by strategy group. A non-parametric comparison of the means
| Group | Strategy | No | Mean No (range) of cells counted | Mann-Whitney U (z value) | p Value |
| 1 | Patients not on eye drops | 10 | 14 (4–57) | 2.88 | 0.004 |
| 2 | Patients on eye drops | 10 | 156 (0–470) | Reference group | |
| 3 | Wiped once | 10 | 9 (2–35) | 2.92 | 0.004 |
| 4 | Wipe/Milton | 10 | 10 (4–35) | 2.95 | 0.003 |
| 5 | Dried 24 hours | 10 | 116 (23–320) | 0.76 | 0.5 |
| 6 | Dried 24 hours/wiped/Milton | 9 | 11 (1–42) | 2.86 | 0.004 |
| 7 | Washed/Milton | 10 | 7 (2–26) | 2.96 | 0.003 |
With Bonferroni correction the results remain significant at the p= 0.05 level.
When the tonometer prism was dried overnight, many cells remained on the surface. Wiping followed by sodium hypochlorite did not remove all of the cells from the surface. There was no difference between the cells recovered before drying, group 2, and after drying, group 5 (z = 0.76, p = 0.48). When the dried tonometer prism was wiped and treated with sodium hypochlorite (group 6), there was a significantly lower cell count compared to group 2 (z = 2.86, p = 0.004). However, there were still some retained cells on the tonometer prism.
Washing and wiping under running water followed by sodium hypochlorite reduced the cell recovery significantly (z = 2.96, p = 0.003); however, it did not completely remove the cellular debris from the tonometer head. Washing under running water was no different from wiping with tissue (z = 1.71, p = 0.08).
The average age of the patients was 74 years (range 45–93). The groups, when compared using a Kruskal-Wallis non-parametric ANOVA with respect to age, were no different (χ2 = 9.6, df = 6, p = 0.2). Of people in groups using eye drops, there were no significant differences in the number of eye drops between the groups (χ2 = 3.2, df = 5, p = 0.7). The average number of eye drops used was 1.7 (range 1–5).
DISCUSSION
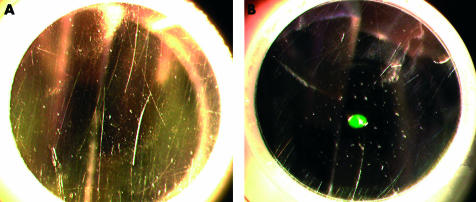
If we take the presence of remnant corneal epithelial cells as a measure of potential prion infectivity of a tonometer prism, it is clear that no current cleaning and disinfection strategy is fully effective. Our study shows that the most important step towards decontamination is the cleaning and mechanical removal of tissue debris. Cleaning has been estimated to reduce infectivity by 4 logs.10 TSE infectivity can be measured using either an LD50 (lethal dose for 50% of the animals)11 or as an ID50 (infectious dose for 50% of the animals).12 However, neither wiping nor rinsing under water removed cellular material completely. Surfaces that appear smooth macroscopically do develop microscopic surface irregularities with time and use (Fig 2). These tonometers, in current use and chosen randomly from our clinics, show that the prism face is corrugated with miniature cracks not evident on macroscopic examination. The applanation surface of the reusable prisms are evaluated grossly by staff, but it was not regular practice before this study to subject the prisms to close scrutiny by examination at the slit lamp before use (as shown in Fig 1). The damaged surface may have trapped debris and this trapping of cellular material could reduce the effectiveness of mechanical cell removal. The baseline slide had three epithelial cells. This may represent contamination by examiners and handlers or dried debris from previous examinations. Because we used reusable tonometer prisms available from the clinic, we cannot exclude contamination from one experiment to the next. The alternative of using disposable tonometer prisms was thought to be less desirable as we wanted to recreate what was actually happening in our clinics. If contamination occurred from test to test then it is also occurring in clinical practice. These tonometers were in current use; they would have been used on these eyes whether the patients had been in the study or not. The patients were not subject to any additional procedure by being in the study.
Figure 2.
Used tonometer prisms, before (A) amd after (B) tonometry.
The amount of retained cellular material following wiping or washing is extremely small (Table 1). However, if the tonometer is not wiped a substantial number of cells remain. In transmissible mink encephalopathy, corneal epithelium was highly infectious.13 Intact corneal epithelium was much more infectious than cultured corneal epithelial cells. The corneal epithelium had an LD50 of 104.8 LD50 per 0.05 ml compared to cultured epithelium whose LD50 was 100.8 with intracerebral injection, suggesting that infectivity might be located in the numerous free nerve endings in the intact corneal epithelium.13 This notion is supported by the finding of neuronal degeneration in the trigeminal ganglia in CJD.14 If we apply this theory to CJD and vCJD it may be that whole cornea or at least intact corneal lamella may be more infectious than desquamated corneal epithelial cells. Of particular interest, in humans, a recent study using a western blot technique found no detectable abnormal prion protein in the cornea from one case of sporadic CJD and one case of vCJD.15 The authors concluded that detection may have been limited by the small amounts of eye tissue available but any PrPsc present in the cornea would be much lower than levels found in other peripheral tissues such as lymphoreticular tissue.
The infective agent in CJD, known as a prion, is a cell membrane protein that has undergone a conformational change from a predominant alpha helix to a proteinase resistant β sheet-rich structure. The prion is highly resistant to inactivation by many techniques of disinfection currently used.1 Methods of sterilisation such as steam autoclaving for 1 hour at 132°C and immersion in 1 M NaOH for 1 hour1 were previously thought to be completely effective,1 but other studies disagree.16,17 Neither is suitable nor practical for the tonometer prism. Agents in current use to disinfect tonometer prisms include sodium hypochlorite at different concentrations (0.05%, 0.1% w/v, 2%), 70% alcohol wipes, sodium dichloroisocyanurate, and 3% hydrogen peroxide. Hydrogen peroxide, isopropyl alcohol, and sodium dichloroisocyanurate are ineffective against TSEs.1,17,18 Solutions of sodium hypochlorite have been shown to be partially or completely effective depending on the amount of free and available chlorine.16,17,19 A 0.5% (w/v) sodium hypochlorite (10 times the strength we used) incubated for 15 minutes with CJD infected guinea pig brain suspension of 5.5–6 log LD50 reduced LD50 by more than 3.5 logs.19 A 30 minute exposure to 13 750 ppm of available chlorine was shown to be completely effective against two strains of BSE in suspension.17 This led to a recommendation that a 1 hour exposure to 20 000 ppm (2%) should be used in practice.17 This forms the basis of the College of Optometrists and Association of British Dispensing Opticians (Guidance on the reuse of Contact Lenses and Ophthalmic Devices, September 2001) recommendation to use of 2% sodium hypochlorite for 1 hour for tonometer prisms and contact lenses. Haag-Streit UK also recommends this concentration and duration (“Cleaning and disinfection of tonometer prisms” Issue 06 01/2000). We chose sodium hypochlorite in this study because of this known advantage over other disinfecting agents. We can make no further conclusions about sodium hypochlorite from out study, merely that its use is supported on theoretical grounds.
Most inactivation studies have been conducted on solutions and homogenates of infectivity and have not dealt with infectivity on surfaces; surface behaviour may be different from infectivity behaviour in solutions. It is known that heat can increase the resistance to disinfection17 but the effect of drying is uncertain. If a tonometer is used without mechanically cleaning, a larger load of potential infectivity would be delivered to the corneal surface with the next measurement as shown in this study. Once the infectious agent is on the cornea, it could theoretically infect the brain via the trigeminal nerve or via the conjunctiva associated lymphatic tissue (CALT).
Does the presence of a few cells on a tonometer prism really represent infectivity? This question cannot be answered fully by this study. However, the potential for cross infection exists, as long as cells remain on tonometer heads. Further work looking for a marker of disease such as abnormal prion protein on tonometer heads following contamination with brain homogenates and decontamination with sodium hypochlorite could answer the question more fully. In the light of our findings, it would be prudent to consider regular slit lamp microscopic examination, tracking, and exchange of reusable tonometer prisms and to avoid using prisms with damaged surfaces. The manufacturers of reusable prisms, Haag-Streit, acknowledge that the prisms have a finite lifespan; however, without routine tracking and monitoring, quality control of prisms may prove difficult. Haag-Streit UK (owner of Clement Clarke) recommends that reusable prisms should be used 100 times only (“Cleaning and disinfection of tonometer prisms” Issue 06 January 2000). As yet this recommendation has not gained widespread clinical practice and further studies demonstrating damage are required.
Other ophthalmic instruments come into contact with the cornea. Corneal burrs remove tissue from the cornea. These are cleaned by weak heat or alcohol and neither is effective against TSE. A burr’s grooved surface renders it difficult to mechanically clean fully between uses; as it cannot be adequately cleaned it should not be reused. The problem of instruments too delicate for harsh decontamination strategies and too expensive to be disposable,20 such as diagnostic and therapeutic contact lenses used for diagnosis and treatment, needs addressing. All coated lenses such as goniolenses, contact fundus lenses, and laser lenses are at risk of degradation with immersion in chemical disinfecting agents.
It is possible to completely eliminate the risk from tonometry by using single use or non-contact methods. Several techniques are currently available: Tonopen (Mentor), disposable tonometer tips (Clement Clarke) and air-puff tonometry. In the study hospital we have adopted the approach of using Tonopen readings almost exclusively apart from in glaucoma patients and to confirm elevated pressure. For reusable Goldmann tonometry, we suggest a multistep approach to reduce CJD risk. Firstly, ophthalmic staff should be educated about and elicit risk factors for CJD and vCJD routinely in clinical practice, especially in higher risk areas such as the United Kingdom. Secondly, our study illustrates the importance of manual cleaning of tonometer heads before disinfection. This is the single most important step in reducing the CJD risk with reusable Goldmann tonometry and should be emphasised to all people performing tonometry.
Acknowledgments
The authors thank G Stanes for the cytology work and staff nurse Z MacKenzie, Princess Alexandra Eye Pavilion, Edinburgh, for assistance in collecting specimens.
REFERENCES
- 1.Lueck CJ, McIlwaine GG, Zeidler M. Creutzfeldt-Jakob disease and the eye. I. Background and patient management. Eye 2000;14:263–290. [DOI] [PubMed] [Google Scholar]
- 2.Rabinstein AA, Whiteman ML, Shebert RT. Abnormal diffusion-weighted magnetic resonance imaging in Creutzfeldt-Jakob disease following corneal transplantations. Arch Neurol 2002;59:637–9. [DOI] [PubMed] [Google Scholar]
- 3.Tateishi J. Transmission of Creutzfeldt-Jakob disease from human blood and urine into mice. Lancet 1985;2:1074. [DOI] [PubMed] [Google Scholar]
- 4.Manuelidis EE, Angelo JN, Gorgacz EJ, et al. Experimental Creutzfeldt-Jakob disease transmitted via the eye with infected cornea. N Engl J Med 1977;296:1334–36. [DOI] [PubMed] [Google Scholar]
- 5.Davanipour Z, Alter M, Sobel E, et al. Creutzfeldt-Jakob disease: possible medical risk factors. Neurology 1985;35:1483–6. [DOI] [PubMed] [Google Scholar]
- 6.Davanipour Z, Goodman L, Alter M, et al. Possible modes of transmission of Creutzfeldt-Jakob disease. N Engl J Med 1984;311:1582–3. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo M, Corbett JJ, Thompson HS. Is applanation tonometry a risk factor for transmission of Creutzfeldt-Jakob disease? Arch Ophthalmol 1987;105:314. [DOI] [PubMed] [Google Scholar]
- 8.Walia JS, Chronister CL. Possible iatrogenic transmission of Creutzfeldt-Jakob disease via tonometer tips: a review of the literature. Optometry 2001;72:649–52. [PubMed] [Google Scholar]
- 9.Zerr I, Brandel JP, Masullo C, et al. European surveillance on Creutzfeldt-Jakob disease: a case-control study for medical risk factors. J Clin Epidemiol 2000;53:747–54. [DOI] [PubMed] [Google Scholar]
- 10.Rutala WA, Weber DJ. Creutzfeldt-Jakob disease: recommendations for disinfection and sterilization. Clin Infect Dis 2001;32:1348–56. [DOI] [PubMed] [Google Scholar]
- 11.Reed LJ, Muench H. A simple method of estimating 50 percent end points. Am J Hygiene 1938:493–7.
- 12.Karber G. Beitrag zur kollectiven Behandlung Pharmakologische Reihen versuche. Arch Exp Pathol Pharmacol 1931:480–3.
- 13.Marsh RF, Hanson RP. Transmissible mink encephalopathy: infectivity of corneal epithelium. Science 1975;187:656. [DOI] [PubMed] [Google Scholar]
- 14.Guiroy DC, Shankar SK, Gibbs CJ Jr, et al. Neuronal degeneration and neurofilament accumulation in the trigeminal ganglia in Creutzfeldt-Jakob disease. Ann Neurol 1989;25:102–6. [DOI] [PubMed] [Google Scholar]
- 15.Wadsworth JD, Joiner S, Hill AF, et al. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet 2001;358:171–80. [DOI] [PubMed] [Google Scholar]
- 16.Taylor DM. Resistance of transmissible spongiform encephalopathy agents to decontamination. In: Rabenau HF, Cinatl J, Doerr HW, eds. Prions. A challenge for science, medicine and public helath system. Basle: Karger, 2001:58–67. [DOI] [PubMed]
- 17.Taylor DM. Inactivation of transmissible degenerative encephalopathy agents: a review. Vet J 2000;159:10–17. [DOI] [PubMed] [Google Scholar]
- 18.Dickinson AG, Taylor DM. Resistance of scrapie agent to decontamination. N Engl J Med 1978;299:1413–14. [DOI] [PubMed] [Google Scholar]
- 19.Brown P, Gibbs CJ Jr, Amyx HL, et al. Chemical disinfection of Creutzfeldt-Jakob disease virus. N Engl J Med 1982;306:1279–82. [DOI] [PubMed] [Google Scholar]
- 20.Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology 2000;55:1075–81. [DOI] [PubMed] [Google Scholar]