Abstract
Background/aims: Ultrastructural alterations in the stroma adjacent to corneal perforations have previously been reported in patients with longstanding rheumatoid arthritis. Since patients with rheumatoid arthritis often present upregulation of proinflammatory cytokines in serum and in synovial fluid, it was of interest to analyse the gene expression of these cytokines—for example, tumour necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), in corneal samples from patients with corneal ulcerations and/or perforations associated with rheumatoid arthritis.
Methods: Corneal samples from seven patients with corneal ulcerations and/or perforations associated with rheumatoid arthritis were collected in 4% paraformaldehyde in “RNAse-free” conditions. Paraffin sections were fixed on silan coated slides and further analysed by systematic non-radioactive in situ hybridisation, using specific gene probes for TNF-α and IL-6 labelled with digoxigenin (DIG). Detection of hybrids was carried out by using a commercially available DIG detection system.
Results: Whereas an extended TNF-α gene expression could be clearly observed in the keratocytes surrounding the corneal ulcerations and/or perforations from five of the seven analysed patients, all seven patients presented clearly positive results for an extended IL-6 gene expression in the analysed tissue samples.
Conclusions: Alterations in corneal cells surrounding ulcerations and/or perforations in patients with rheumatoid arthritis may occur with implication for inflammatory processes. Upregulation of the proinflammatory cytokines TNF-α and IL-6 may modify the production of metalloproteinases in the corresponding cells resulting in collagenolytic corneal damage.
Keywords: rheumatoid arthritis, proinflammatory cytokines, keratocytes, in situ hybridisation
Patients with rheumatoid arthritis often present with ocular complications such as aqueous tear deficiency, scleritis, and central or paracentral corneal ulceration.1–7 Moreover, the occurrence of ultrastructural alterations in the stroma adjacent to non-inflammatory corneal perforations associated with longstanding rheumatoid arthritis has also been reported.8 As described earlier,8 it is possible to find electron dense deposits (possibly aggregated collagen) in the extracellular stromal matrix surrounding the corresponding perforation. However, light microscopy examination of the same tissue samples revealed only a few inflammatory cells surrounding the perforation, which would indicate the lack of clinically detectable inflammation in these patients. In fact, it seems to be widely accepted that there is little or no clinically detectable inflammation on the ocular surface of such patients, as deduced by using classic and/or conventional examination methods. If true, this preliminary statement had to be corroborated by using other available methods of examination—for example, at the molecular level. However, just by using systematic RNA in situ hybridisation analysis, we could detect high levels of RNA gene expression for proinflammatory cytokines such as tumour necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) in the keratocytes from patients with rheumatoid corneal ulcerations. This finding may first indicate the implication of widely extended inflammatory processes in the corresponding corneal cells from patients with corneal ulcerations and/or perforations associated with rheumatoid arthritis.
MATERIALS AND METHODS
Corneal samples from seven patients with corneal ulcerations and/or perforations associated with rheumatoid arthritis were collected and stored in 4% paraformaldehyde before being paraffin embedded in RNAse-free conditions. From all the collected samples, 4–5 μm paraffin sections were sequentially obtained in RNAse-free conditions and further fixed on RNAse-free silan coated slides. The resulting paraffin sections were then analysed by systematic non-radioactive in situ hybridisation also performed in strict RNAse-free conditions. Alternatively, some of the paraffin sections were routinely stained with haematoxylin and eosin. In the present study, the in situ hybridisations were carried out with specific gene probes for TNF-α and IL-6 labelled with digoxigenin (DIG) by using the DIG labelling kit from Boehringer-Mannheim (Mannheim, Germany). The whole hybridisation procedure was performed as described elsewhere,9–13 including all the corresponding control hybridisations and control samples as previously described.9–13 In addition, control samples obtained from two patients with keratoconus were tested in the same conditions as those from the patients with rheumatoid arthritis. Detection of hybrids was carried out with the DIG detection system from Boehringer-Mannheim, which provides a violet blue colour reaction on the positive cells, after the addition of the corresponding substrates for alkaline phosphatase and incubation for at least 24 hours at 4°C. Evaluation of positive or negative hybridisation results in the analysed samples was systematically carried out by using the hybridisation score system presented in Table 1, and was initially performed without any sample reference. Furthermore, the same evaluation procedure was repeated at least three times, and representative results were finally photographically documented. The whole evaluation procedure was performed by using a light microscope (Axiophot, Zeiss, Germany).
Table 1.
TNF α and IL-6 gene expression in keratocytes from patients with corneal ulcerations and/or perforations associated with rheumathoid arthritis*
| Patient No | Age (years) and sex | Ulceration and transplantation problematic | Systemic manifestation | TNF α gene expression | IL-6 gene expression |
| 1 | 59 (F) | paracentral perforated keratomalacia | rheumatoid arthritis | +++ | ++ |
| 2 | 76 (F) | perforation | rheumatoid arthritis | + | + |
| 3 | 60 (F) | central descemetocele | rheumatoid arthritis† +herpes zoster | – | ++ |
| 4 | 58 (F) | ring ulcer + sicca syndrome | rheumatoid arthritis +Sjögren syndrome | +++ | ++ |
| 5 | 74 (F) | peripheral perforation, thinning | rheumatoid arthritis, osteoporosis + diabetes mellitus | ++ | + |
| 6 | 72 (M) | central keratitis | rheumatoid arthritis† +Staphylococcus sp | – | ++ |
| 7 | 71 (F) | peripheral keratitis descemetocele | rheumatoid arthritis | ++ | + |
*Results observed by specific non-radioactive in situ hybridisations, compared to the clinical data of the patients before surgery. Score used for evaluation of the in situ hybridisation results: (–) negative/not detected, <5% positive cells, (+) clearly positive reaction, approximately 5–10% positive cells, (++) extended positive reaction, 10–20% positive cells, (+++) very extended positive reaction, 20–50% or more positive cells.
†Rheumatic symptoms not definitively confirmed.
RESULTS
A summary of relevant clinical data from the seven patients with corneal ulcerations and/or perforations is presented in Table 1. Both DIG labelled gene probes for TNF-α and IL-6 yielded clearly positive results for the presence of high levels of the corresponding mRNA molecule by using the performed insitu hybridisation procedure in the analysed corneal samples. Whereas an extended TNF-α gene expression could be clearly observed in the keratocytes surrounding the corresponding corneal ulcerations and/or perforations from five of the seven analysed patients, all seven patients presented clearly positive results for an extended IL-6 gene expression mainly observed in the keratocytes from the same tissue samples.
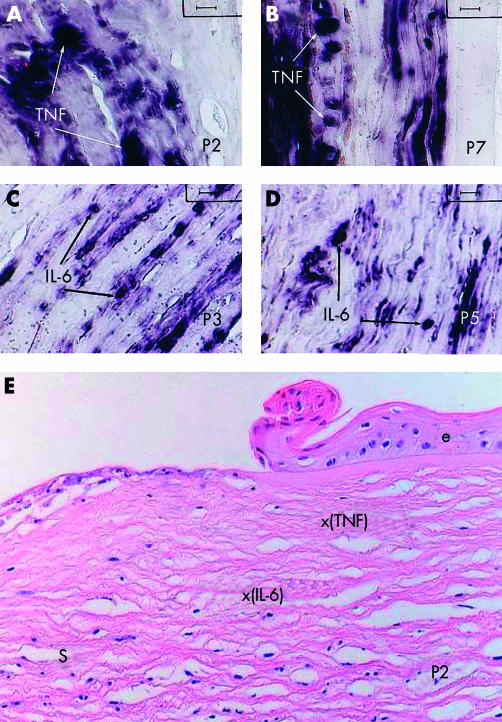
Representative TNF-α mRNA hybridisation patterns, corresponding to the patients 2 and 7, are shown in Figure 1A and B, respectively. Furthermore, in a similar manner, representative IL-6 mRNA hybridisation patterns, corresponding to the patients 3 and 5, are shown in Figure 1C and D, respectively. Moreover, whereas the keratocytes showing a clear positive reaction for TNF-α, mRNA hybridisation were more often localised in the areas surrounding the corneal epithelium (see Fig 1A, B, and E), the cells which showed a positive reaction for IL-6 mRNA hybridisation were mainly found deeper in the stroma, and often in groups of long series (chains) of activated cells (see Fig 1C, D, and E). The results for TNF-α and IL-6 in situ hybridisations are further summarised in Table 1, which provides semiquantitative data about the observed gene expression of the analysed cytokines for each series of screened samples. Interestingly, the two patients with non-detectable TNF-α gene expression (patients 3 and 6, both with score: “–”) presented both central corneal ulcerations and additional acute infections (herpes zoster in patient 3 and Staphylococcus sp in patient 6). Furthermore, no clear rheumatic symptoms could be confirmed in these two patients with missing TNF-α gene expression in the corneal samples.
Figure 1.
Detection of TNF-α (A, B, and E) and IL-6 (C, D, and E) mRNA by non-radioactive in situ hybridisation in corneal sections from patients with corneal ulcerations and/or perforations associated with rheumatoid arthritis. (A) Representative TNF-α mRNA hybridisation pattern of the corneal keratocytes in a paraffin section from patient 2 (hybridisation score: +). (B) Representative TNF-α mRNA hybridisation pattern of the corneal keratocytes in a paraffin section from patient 7 (hybridisation score: ++). (C) Representative IL-6 mRNA hybridisation pattern of the corneal keratocytes in a paraffin section from patient 3 (hybridisation score: ++). (D) Representative IL-6 mRNA hybridisation pattern of the corneal keratocytes in a paraffin section from patient 5 (hybridisation score: +). (E) Representative corneal section from patient 2, stained with haematoxylin and eosin showing the relative position of the stroma cells yielding positive mRNA hybridisations either for TNF-α (x(TNF)), or for IL-6 (x(IL-6)). A positive reaction is indicated by a dark blue-violet colour as a result of the corresponding reaction using the digoxigenin detection kit from Boehringer-Mannheim. TNF = TNF-α, e = corneal epithelium, s = corneal stroma (original magnification (A–D) ×1000, (E) ×200).
In addition, when control samples obtained from patients with keratoconus were tested for mRNA in situ hybridisation, neither TNF-α nor IL-6 gene expression could be detected in the same conditions.
DISCUSSION
The results clearly show that both inflammatory cytokines TNF-α and IL-6 are widely expressed in the keratocytes of patients with corneal ulcerations and/or perforations associated with rheumatoid arthritis. The obtained mRNA hybridisation results may further indicate that not only TNF-α (detected in 70% of the analysed patients) but also IL-6 (detected in 100% of the analysed patients) often seem to be implicated in the observed corneal ulcerations and/or perforations. Moreover, the two observed cases of missing TNF-α gene expression were, interestingly, found to be associated with additional acute infections (herpes zoster or Staphylococcus sp) as well as with non-confirmed symptoms for rheumatoid arthritis. The detected high levels of TNF-α and IL-6 gene expression in the analysed corneal samples directly correspond with the in vivo conditions during corneal ulceration and/or perforation shortly before surgery, since the collected samples were immediately fixed in RNAse-free 4% paraformaldehyde, even during surgery. However, additional patient dependent factors, like their immune status, and/or possible effects of actual or previous treatments, may also have modified the observed cytokine gene expression and additional studies are needed in order to characterise these additional effects. IL-6 is widely known to promote epithelial migration by a fibronectin dependent mechanism,14 and furthermore has been found to stimulate collagen synthesis and to reduce the production of metalloproteinases in human keratocytes.15 In contrast, the role of TNF-α has more often been associated with pathological and degenerative processes in a wide variety of cells.16,17 It has been proposed that TNF-α may be associated with extensive cell death (apoptosis) and nuclear degeneration in the lens of developing avian embryos,18 but additional studies need to be performed with human samples in order to confirm this effect in the corresponding human cells. The results indicate that alterations in corneal cells surrounding ulcerations and/or perforations associated with rheumatoid arthritis may occur with implication of inflammatory processes. Upregulation of the proinflammatory cytokines TNF-α and IL-6 may modify the production of metalloproteinases in the corresponding cells, resulting in collagenolytic corneal damage. Currently, we can conclude that the inflammatory cytokines IL-6 and TNF-α are both probably produced by keratocytes of the analysed patients, representing one of the main causes or consequences of corneal ulceration and perforation associated with rheumatoid arthritis.
REFERENCES
- 1.McGavin DDM, Williamson J, Forrester JV, et al. Episcleritis and scleritis. A study of their clinical manifestations and association with rheumatoid arthritis. Br J Ophthalmol 1976;60:163–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feder RS, Krachmer JH. Conjunctival resection for the treatment of rheumatoid corneal ulceration. Ophthalmology 1984;91:111–15. [DOI] [PubMed] [Google Scholar]
- 3.Gardner KM, Rajacich GM, Mondino BJ. Ophthalmological manifestations of adult rheumatoid arthritis and cicatricial pemphigoid. Int Ophthalmol Clin 1985;25:1–35. [DOI] [PubMed] [Google Scholar]
- 4.Kervick GN, Pflugfelder SC, Haimovici R, et al. Paracentral rheumatoid corneal ulceration. Clinical features and cyclosporine therapy. Ophthalmology 1992;99:80–8. [DOI] [PubMed] [Google Scholar]
- 5.Saal JG, Fritz P, Zymela B, et al. Immunhistologische und Immunhistochemische Untersuchungen zur Pathogenese der Keratomalazie bei rheumatoider Arthritis. Z Rheumatol 1991; 50:151–9. [PubMed] [Google Scholar]
- 6.Pleyer U, Bergmann L, Krause A, et al. Autoimmunerkrankungen der peripheren Hornhaut. Immunpathologie, Klinik und Therapie. Klin Monatsbl Augenheilk 1996;208:73–81. [DOI] [PubMed] [Google Scholar]
- 7.Pleyer U, Bertelmann E, Rieck P, et al. Outcome of penetrating keratoplastic in rheumatoid arthritis. Ophthalmologica 2002;216:249–53. [DOI] [PubMed] [Google Scholar]
- 8.Adachi W, Nishida K, Quantock AJ, et al. Ultrastructural alterations in the stroma adjacent to non-inflammatory corneal perforations associated with long standing rheumatoid arthritis. Br J Ophthalmol 1998;82:1445–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pringle JH, Primrose L, Kind CN, et al. In situ hybridization demonstration of poly-adenylated RNA sequences in formalin-fixed paraffin sections using a biotinylated oligonucleotide poly d(T) probe. J Pathol 1989;158:279–86. [DOI] [PubMed] [Google Scholar]
- 10.Flemming KA, Evans M, Ryley KC, et al. Optimization of non-isotopic in situ hybridization on formalin-fixed paraffin-embedded material using digoxigenin-labelled probes and transgenic tissues. J Pathol 1992;167:9–17. [DOI] [PubMed] [Google Scholar]
- 11.Prada J, Neifer S, Müller S, et al. Splenic interleukin 1 gene expression is associated with accumulation of macrophages and oxygen radical production in Plasmodium vinckei malaria. J Pathol 1994;174:57–62. [DOI] [PubMed] [Google Scholar]
- 12.Prada J, Malinowski J, Müller S, et al. Hemozoin differentially modulates the production of interleukin 6 and tumor necrosis factor in murine malaria. Eur Cytokine Netw 1995;6:109–12. [PubMed] [Google Scholar]
- 13.Prada J, Ngo-Tu T, Baatz H, et al. Detection of tumor necrosis factor and interleukin 1 alpha gene expression in human lens epithelial cells. J Cataract Refract Surg 2000;26:114–17. [DOI] [PubMed] [Google Scholar]
- 14.Nishida T, Nakamura M, Mishima H, et al. Interleukin 6 promotes epithelial migration by a fibronectin-dependent mechanism. J Cell Physiol 1992;153:1–5. [DOI] [PubMed] [Google Scholar]
- 15.Malecaze F, Simorre V, Chollet P, et al. Interleukin 6 in tear fluid after photorefractive keratectomy and its effects on keratocytes in culture. Cornea 1997;16:580–7. [PubMed] [Google Scholar]
- 16.Le J, Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest 1987;561:234–48. [PubMed] [Google Scholar]
- 17.Beutler B, Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med 1987;316:379–85. [DOI] [PubMed] [Google Scholar]
- 18.Sanders EJ, Wride MA. Roles for growth and differentiation in avian embryonic development. Poult Sci 1997;76:111–17. [DOI] [PubMed] [Google Scholar]