Abstract
Aim: To prospectively assess the efficacy and complications of viscocanalostomy with a reticulated hyaluronic acid implant (VSRHAI) versus standard viscocanalostomy in patients with medically uncontrolled open angle glaucoma.
Methods: A consecutive series of 40 patients (40 eyes) with uncontrolled open angle glaucoma underwent non-penetrating antiglaucomatous surgery. After the excision of the deep scleral flap they were randomly assigned to either a standard viscocanalostomy or additional implantation of a reticulated hyaluronic acid implant. Follow up visits were over a period of 12 months after surgery.
Results: The mean preoperative intraocular pressure (IOP) was 26.5 (SD 6.1) mm Hg for all patients enrolled. The mean IOP was 8.1 (SD 5.6) mm Hg 1 day after surgery for the viscocanalostomy group (p<0.001) and 12.0 (SD 5.2) mm Hg for the VSRHAI group (p<0.001). The postoperative IOP difference between the two groups was statistically significant (p = 0.03). The success rate, defined as an IOP lower than 22 mm Hg without medication, was 40% in both groups at 12 months postoperatively (p = 0.90). The number of postoperative complications was equally low for both groups.
Conclusions: Both surgical procedures, viscocanalostomy and VSRHAI, provide comparable success rates over a 1 year follow up period. The specific intraoperative and postoperative complications of non-penetrating surgery were seen in our series, although the overall rate of postoperative complications proved equally low for both techniques.
Keywords: viscocanalostomy, deep sclerectomy, reticulated hyaluronic acid implant, SKGEL, non-penetrating surgery, open angle glaucoma
During recent years the role of non-penetrating surgery in the management of glaucoma has become one of the most discussed topics among ophthalmic surgeons. The non-penetrating procedures are designed to avoid full thickness penetration into the anterior chamber, aiming to overcome the risk of severe postoperative complications due to overfiltration and hypotony which are well known from trabeculectomy. Several studies have shown that lowering of the intraocular pressure (IOP) can be achieved by non-penetrating antiglaucomatous surgery.1–5 Although the exact IOP reduction mechanism following these procedures is still not well defined, the intrascleral space created by deep sclerectomy is thought to be of superior significance for the transport of aqueous into the perilimbal drainage channels.
Variants of the basic non-penetrating surgical technique were developed to protect the intrascleral “decompression” space from scarring with subsequent loss of IOP control. Viscocanalostomy, first described by Stegmann in 1991, differs from basic deep sclerectomy in that Schlemm’s canal and the intrascleral space are injected with high molecular weight sodium hyaluronate. Early reports on the antiglaucomatous efficacy of viscocanalostomy were encouraging.6 However, recent reports indicate that viscocanalostomy does not reach standard trabeculectomy in terms of IOP reduction and success rate.5,7 A different development led to intrascleral implants made of collagen (Aqua-Flow, Staar Surgical AG, Nidau, Switzerland)8–10 or reticulated hyaluronic acid. A recent retrospective pilot study of deep sclerectomy with implantation of reticulated hyaluronic acid into the intrascleral space demonstrated good results.11
In theory the reticulation of the hyaluronate hinders its rapid degradation, thereby preventing the intrascleral lake from collapse and fibrosis. Although the potential advantages of this cross linked hyaluronic acid preparation have been repeatedly emphasised, there is still no significant evidence for its superiority to the classic non-reticulated sodium hyaluronate which is used during viscocanalostomy.
The lack of substantial prospective data comparing these non-penetrating antiglaucomatous techniques caused us to initiate a randomised trial to assess the efficacy and risk profile of viscocanalostomy with the reticulated hyaluronic acid implant (VSRHAI) compared with viscocanalostomy in patients with medically uncontrolled open angle glaucoma.
METHODS
A consecutive series of 40 patients (40 eyes) with uncontrolled open angle glaucoma underwent non-penetrating antiglaucomatous surgery. Patients were included in the study if they had primary or secondary open angle glaucoma with uncontrolled IOP while receiving maximal tolerable antiglaucomatous therapy. Patients with angle closure glaucoma, post-traumatic, uveitic, neovascular, or dysgenetic glaucoma were not considered for this study. Patients with a history of previous ocular surgery and younger than 21 years of age were excluded from the study. Possible alternatives, beneficial effects, and potential complications of the surgical procedure were explained in detail to all patients. Written informed consent was obtained from all participants. Before surgical intervention all patients underwent a baseline examination which included measurement of best corrected visual acuity (ETDRS charts, Lighthouse, Long Island, USA), visual field examination (30-2, Humphrey field analyser, Humphrey Instruments, Munich), biomicroscopy, gonioscopy, and Goldmann applanation tonometry.
All operations were performed as follows. The procedure commenced with the creation of a fornix based conjunctival flap. After the dissection of a superficial limbus based triangular scleral flap, a second limbus based scleral flap was carefully dissected beneath the previous one towards the choroid. Schlemm’s canal was deroofed during the extension of the deep scleral flap to its limbal edges. After probing the artificial orifices, Schlemm’s canal was delicately filled with high molecular weight sodium hyaluronate (Healon GV, Pharmacia, Groningen, Netherlands). The deeper flap was then excised and the patients were assigned randomly to receive either a standard viscocanalostomy or implantation of a reticulated hyaluronic acid implant (SKGEL 3.5, Corneal, Paris, France). Whenever the procedure was continued as viscocanalostomy the superficial scleral flap was tightly closed with two 10-0 nylon sutures (10-0, CU-1, Alcon Surgical, Inc, Houston, TX, USA) and the viscoelastic substance was carefully injected under the scleral flap. When reticulated hyaluronic acid was needed the triangular implant was positioned into the scleral bed (see Fig 3). The superficial scleral flap was then carefully repositioned and tightly closed with two 10-0 nylon sutures. Finally the conjunctiva was closed with a running 9-0 polyglactin suture (Vicryl, Ethicon Limited, Edinburgh, UK). The standard postoperative regimen consisted of topical gentamicin and dexamethasone five times daily.
Figure 3.
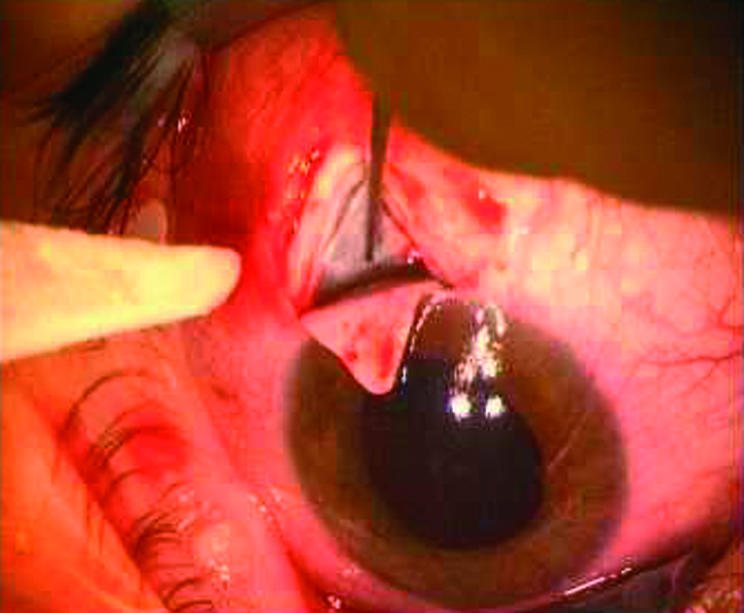
Intraoperative situs showing the SKGEL implant inside the scleral bed.
When a visible perforation of the trabeculodescemetic membrane occurred during the dissection of the deep scleral flap, a guarded penetrating procedure was used. Postoperatively, examinations were performed on a daily base for 1 week. Follow up visits were applied 0.5, 1, 3, 6, 9, and 12 months after surgery. At each follow up visit all the aforementioned examinations except visual field testing were repeated. The visual field examination was repeated at 6 and 12 months after surgery. Adjunctive subconjunctival 5-fluorouracil injections and laser goniopuncture were not applied during the follow up period.
Complete success was defined as IOP < 22 mm Hg without any additional glaucoma surgery or medication whereas qualified success was defined as IOP <22 mm Hg with additional antiglaucoma medications. Particular attention was paid to postoperative complications.
Statistical analysis were performed using the spss 10.0 software package (SPSS Inc, Munich, Germany). Student’s t test was used to compare means. The χ2 test was used for comparison of the qualitative data between the two groups. Kaplan-Meier survival curves were drawn. Intercurve analysis was performed using the log rank test.
RESULTS
Forty (20/20) patients, 33 men and seven women, completed the study. The mean age was 60.8 (SD 18.1) years. Thirty nine patients (97.5%) were white and one black African. Twenty seven patients had primary open angle glaucoma and 13 patients had secondary open angle glaucoma, of whom eight patients with pseudoexfoliation glaucoma and five eyes with pigmentary glaucoma. In two eyes an inadvertent visible perforation of the trabeculodescemetic membrane without iris plugging occurred during the dissection of the deep scleral flap. In one eye the dissection of the deep scleral flap was performed without complications, but a definite identification of Schlemm’s canal was not possible. In these three eyes the surgeon switched over to a guarded filtering procedure. These patients were excluded from the study and replaced. The mean preoperative intraocular pressure (IOP) was 26.5 (SD 6.1) mm Hg for all patients enrolled. No difference with respect to the mean preoperative IOP was observed between the two groups (p = 0.74).
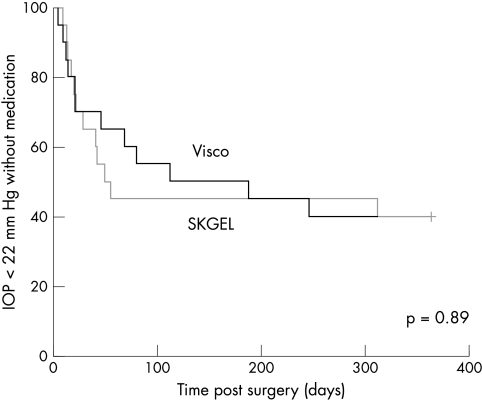
One day after surgery the mean postoperative IOP was 8.1 (SD 5.6) mm Hg for the viscocanalostomy group (p<0.001) and 12.0 (SD 5.2) mm Hg for the VSRHAI group (p<0.001), respectively. During the first week after surgery the viscocanalostomy group showed a lower mean IOP than the VSRHAI group, which was only statistically significant on the first postoperative day (p=0.03) (Table 1). Survival analysis revealed no significant difference between the two groups with reference to both success criteria. At 12 months after surgery, the complete success rate, defined as an IOP lower than 22 mm Hg without medication, was 40% in both groups (p=0.90) (Fig 1). Additionally no difference in terms of qualified success was observed between the applied surgical techniques. In both groups qualified success was achieved in 85% of patients (p=0.99).
Table 1.
Mean IOP and mean number of antiglaucoma medications during the postoperative course after viscocanalostomy/VSRHAI.
| Viscocanalostomy | VSRHAI | p Value (t test) | ||||
| Mean IOP (mm Hg) (SD) | Mean number of medications (SD) | Mean IOP (mm Hg) (SD) | Mean number of medications (SD) | IOP | Meds | |
| Preop | 26.8 (6.4) | 3.0 (1.1) | 26.2 (6.0) | 2.80 (1.1) | 0.74 | 0.72 |
| Postop 1 | 8.1 (5.6) | – | 12.0 (5.2) | – | 0.03 | – |
| Postop 3 | 8.7 (6.5) | – | 12.3 (9.1) | – | 0.18 | – |
| Postop 7 | 10.2 (6.1) | – | 11.8 (4.4) | – | 0.37 | – |
| FU 1/2 | 13.2 (6.8) | 0.1 (0.5) | 15.8 (5.8) | 0.1 (0.2) | 0.28 | 0.6 |
| FU 1 | 16.0 (4.4) | 0.4 (0.9) | 17.8 (4.0) | 0.3 (0.6) | 0.22 | 0.76 |
| FU 3 | 15.4 (4.3) | 0.5 (1.3) | 16.4 (3.3) | 0.5 (0.8) | 0.49 | 0.93 |
| FU 6 | 16.4 (6.5) | 0.5 (0.9) | 15.9 (2.4) | 0.6 (1.0) | 0.78 | 0.64 |
| FU 9 | 14.7 (3.5) | 0.8 (1.1) | 15.1 (2.7) | 0.8 (1.1) | 0.70 | 1 |
| FU 12 | 16.5 (5.8) | 0.7 (1.0) | 17.0 (3.1) | 0.6 (1.0) | 0.75 | 0.91 |
FU 1 = follow up examination 1 month after surgery; post op 1 = examination 1 day after surgery; IOP = intraocular pressure; Meds = antiglaucoma medications; SD = standard deviation, mean = mean value of those patients that fullfilled at least the qualified success criteria.
Figure 1.
Kaplan-Meier cumulative survival curves for an IOP of less than 22 mm Hg without additional antiglaucomatous medications (complete success). Viscocanalostomy patients, thick line, VSRHAI patients, thin line. Intercurve analysis using the log rank test revealed no significant difference between the two groups (p=0.89).
Post hoc power calculation including a median survival time of 49 days for the VSRHAI group and of 111 days for the viscocanalostomy group revealed a value of 0.83. This strongly indicates that there is truly no statistical difference between the two treatments.
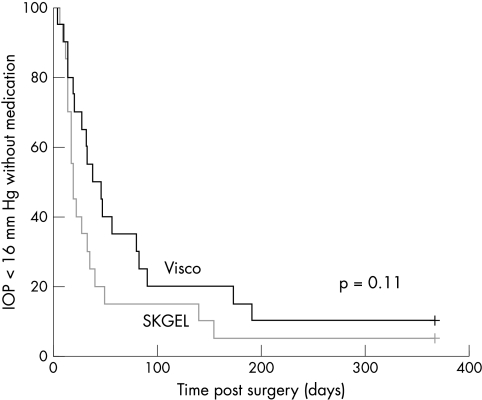
As the targets of intraocular pressure are becoming increasingly stringent a survival analysis with reference to a target pressure of less than 16 mm Hg was additionally calculated (Fig 2). Even so this analysis did not bring out any difference between the surgical procedures (p=0.11).
Figure 2.
Kaplan-Meier cumulative survival curves for an IOP of less than 16 mm Hg without additional antiglaucomatous medications. Viscocanalostomy patients, thick line, VSRHAI patients, thin line. Intercurve analysis using the log rank test revealed no significant difference between the two groups (p=0.11).
Postoperatively, the mean number of antiglaucoma medications was significantly reduced in both groups. However, there was no statistically significant difference between the two groups at each postoperative visit. At 12 months the mean number of antiglaucoma medications was 0.7 (SD 1.0) in the viscocanalostomy group and 0.6 (SD 1.0) in the VSRHAI group (p=0.96) (Table 1). Compared to the baseline examination, the best corrected visual acuity and mean defect of visual field remained unchanged at the 12 month follow up visit in each group.
The frequency of complications for each group is listed in Table 2. Interestingly, bleb formation was seen in 13 eyes (65%) of the VSRHAI group and in 11 eyes (55%) after viscocanalostomy (p=0.52). In none of the eyes was significant cataract progression noted throughout the follow up period. Postoperatively a transient bleb leakage was seen in two eyes after viscocanalostomy. In one eye of the VSRHAI group resuturing of the conjunctiva was necessary owing to a persistent bleb leakage in the early postoperative period.
Table 2.
Incidence and percentage for each complication recorded.
| Viscocanalostomy | VSRHAI | ||||
| Complication | Patients | (%) | Patients | (%) | χ2 test p Value |
| Perforation of descemet | 2/43* | 4.7 | 2/43* | 4.7 | – |
| Non-identification of canal | 1/43* | 2.3 | 1/43* | 2.3 | – |
| Choroidal deroofing | 2/43* | 4.7 | 2/43* | 4.7 | – |
| Hyphaema | 3/20 | 15 | 2/20 | 10 | 0.63 |
| Hypotony | 6/20 | 30 | 5/20 | 25 | 0.72 |
| Choroidal detachment | 0/20 | 0 | 1/20 | 5 | 0.31 |
| Shallow anterior chamber | 3/20 | 15 | 2/20 | 10 | 0.59 |
| Resuturing of conjunctiva | 0/20 | 0 | 1/20 | 5 | 0.31 |
| Exposure of Skgel implant | NA | NA | 1/20 | 5 | – |
| Cataract progression | 0/20 | 0 | 0/20 | 0 | – |
| Bleb formation | 11/20 | 55 | 13/20 | 65 | 0.52 |
Statistical significance (χ2 test) for differences between groups. NA = not applicable, VSRHAI = deep sclerectomy with a reticulated hyluronic acid implant, *percentage calculated with respect to all procedures performed including those three patients which were excluded.
Conjunctival dehiscence with partial extrusion and exposure of the reticulated hyaluronic acid implant was observed in one patient of the VSRHAI group 2 weeks after the operation (see Fig 3). Topical gentamicin ointment was applied three times daily for the following week. The patient was then seen every other day. A gradual limbal readaptation of the conjunctiva over the surface of the exposed parts of the implant with formation of a filtration bleb was noted. Further follow up visits revealed a controlled IOP without additional medications.
In one eye of the VSRHAI group slight scleral ectasia adjacent to the site of sclerectomy was first observed at the 6 months visit. At the following visits, 9 and 12 months after surgery, no further progression of the ectatic lesion was noted.
DISCUSSION
The non-penetrating surgical procedures allow percolation of aqueous humour through the thinned trabeculodescemetic membrane into the intrascleral space. According to Stegmann’s theory the aqueous humour reaches Schlemm’s opened canal which drains into the episcleral veins. By leaving the trabeculodescemetic membrane intact sudden IOP drops due to overfiltration are avoided. Theoretically, the frequency of postoperative complications encountered with trabeculectomy should be significantly lowered. Variants of the non-penetrating surgery include viscocanalostomy and deep sclerectomy with or without an intrascleral implant.4,6,11,12
Viscocanalostomy was first proposed by Stegmann in 1991. In 1999 encouraging results from a large series of patients treated with viscocanalostomy were reported.6 The study involved 214 eyes of 157 African patients with open angle glaucoma. A postoperative IOP of 22 mm Hg or less without additional antiglaucoma medications was achieved in 83% of eyes with an average follow up of 35 months. Few complications were observed in this series. In contrast with these results recent data from prospective trials indicate lower success rates of less than 40% at 1 year after viscocanalostomy.5,7,13
Fyodorov and Kozlov first described a modified type of deep sclerectomy in 1989.8,9 After excision of the deep scleral flap they positioned a collagen implant into the scleral bed. Further research led to an implant made out of lyophilised porcine scleral collagen (Aqua-Flow, Staar Surgical AG, Nidau, Switzerland). A recent study reported improved results when this collagen device was used during deep sclerectomy.10 However, there is also evidence from a retrospective study that the implant does not improve the tonometric outcome in primary open angle glaucoma.14 Furthermore, several reports on the outcome after deep sclerectomy with a reticulated hyaluronic acid implant are available. This technique was initially reported by Sourdille in 1999.11 The results of his retrospective study included a complete success rate of 72% after a mean follow up period of 13.8 months. Recently, data from a prospective trial revealed a success rate without medication of 57% after a mean follow up of 11.4 months.15 The results of another recent retrospective study reported a success rate of 80% without medications at 12 months after deep sclerectomy with a reticulated hyaluronic acid implant.16
The latter techniques have in common that the intrascleral space is filled with a more or less absorbable substance which is thought to protect the residual space from fibrosis. Thus, the question arises which substance is best to preserve the “decompression” space and thereby postoperative IOP control. The reticulated structure of the hyaluronic acid implant is thought to considerably slow the physiological breakdown of the hyaluronate within the scleral bed. In histological specimens from rabbit eyes remnants of the hyaluronic acid implant have been seen up to 56 days after surgery.11 Substantial data concerning the postoperative duration of the intrascleral degradation of the implant in human eyes are lacking as such in vivo data in humans are naturally difficult to obtain. As an indirect parameter for the degradation of the reticulated hyaluronic acid implant Marchini—by means of ultrasound biomicroscopy—repeatedly measured the dimensions of the intrascleral space in a series of patients who underwent VSRHAI. No changes with respect to the dimensions of the intrascleral space were seen for a period of at least 3 months after surgery.15
Our present study revealed comparable success rates for deep sclerectomy with a reticulated hyaluronic acid implant and standard viscocanalostomy in eyes with open angle glaucoma. The complete success rate of 40% after viscocanalostomy favourably compares with the results of a recent prospective study comparing viscocanalostomy and trabeculectomy as primary procedures in open angle glaucoma.7 These results indicate that neither viscocanalostomy with a reticulated hyaluronic acid implant nor standard viscocanalostomy offer IOP reduction and success rates that are comparable with standard trabeculectomy.
Various specific intraoperative complications and difficulties of non-penetrating antiglaucomatous surgery have been previously observed.4,7,17,18 Specific complications such as choroidal deroofing, inadvertent perforation of the trabeculodescemetic membrane, and non-identification of Schlemm’s canal were seen among the patients in our trial. Other specific but rare complications of viscocanalostomy, which have recently been reported, include detachment of Descemet’s membrane and scleral ectasia.19,20 The latter was seen in one patient of the VSRHAI group of our series. Overall, mainly mild complications were seen in both groups. The incidence of complications due to overfiltration was slightly lower in the VSRHAI group. Additionally, within the viscocanalostomy group one patient required surgical revision due to a persistent hypotony.
A relatively high rate of bleb formation in both groups was observed although the sutures of the scleral flap had been carefully tightened during surgery. Invisible microperforations and loosening of the superficial scleral flap might have caused the observed subconjunctival filtration. In contrast with our observations, Stegmann reported formation of a filtering bleb in 5% of eyes after viscocanlostomy.6 However, our observation is comparable with the results of Mermoud18 and Marchini15 who both observed a bleb formation in 50% and 60% of eyes after viscocanalostomy and deep sclerectomy with a reticulated hyaluronic acid implant, respectively. Most of the authors15,16 using the SKGEL implant close the scleral flap with two sutures. To allow a certain compatibility with previous reports we also used two sutures for readaptation of the flap. Although others21 close scleral flaps with at least three sutures, they also observe bleb formation in up to 50% of eyes after viscocanalostomy. Thus, it remains at least questionable if a watertight closure of the superficial scleral flap can be achieved even with the use of more than two sutures. Late bleb related complications such as bleb infection and endophthalmitis were not observed in our series.
The mechanism of action by which IOP is reduced by non-penetrating antiglaucomatous techniques still remains to be clarified. Most studies suggest that the abnormal increase in outflow resistance of the glaucomatous eye is found in the juxtacanalicular trabecular meshwork, which is meant to remain unaffected during deep sclerectomy. According to Stegmann’s theory the aqueous humour percolates through the thinned trabeculodescemetic into the intrascleral space which allows the aqueous humour to enter the open ends of Schlemm’s canal and drains into the perilimbal collector channels. Thus, it is thought that the efficacy of non-penetrating antiglaucomatous surgery does not depend on a functioning filtering bleb. It has been shown in monkey eyes that the injection of a viscoelastic substance into Schlemm’s canal can easily cause ruptures of the inner and outer endothelial walls of the canal.22 Additionally, during unroofing of Schlemm’s canal by the perilimbal dissection of the deep scleral flap inadvertent damage to the inner wall of the canal is likely to occur.23 Following these results Johnson and Johnson recently hypothesised that viscocanalostomy functions as a “gentle trabeculectomy.”24
Although Nd:YAG laser goniopuncture has been put forward as an efficient and safe adjunct after deep sclerectomy where filtration through the trabeculodescemetic membrane is considered to be insufficient,25 it perforates the thin trabeculodescemetic membrane, thereby turning the initial operation into a penetrating procedure. As the authors intended to compare the antiglaucomatous effects and the safety profile of two non-penetrating procedures adjunctive Nd:YAG laser goniopuncture was not applied. In accordance with the original technique of viscocanalostomy additional surgical adjuncts, such as a systematic peeling of the inner wall of Schlemm’s canal and juxtacanalicular trabecular tissue,11,15 were avoided because from our own experience this easily leads to perforation. However, aqueous drainage via a seemingly intact trabeculodescemetic membrane just like in our series might be to weak to prevent the intrascleral space from scarring even with the use of an implant. This might explain both the high failure rate in each of the groups of our study and the lack of difference between the two treatments.
In conclusion, both surgical procedures, viscocanalostomy and VSRHAI, offer IOP reduction and success rates which are comparable over a 1 year follow up period. The specific intraoperative and postoperative complications of non-penetrating surgery were seen in our series, although the overall rate of postoperative complications proved equally low for both techniques.
REFERENCES
- 1.Zimmerman TJ, Kooner KS, Ford VJ, et al. Effectiveness of nonpenetrating trabeculectomy in aphakic patients with glaucoma. Ophthalmic Surg 1984;15:44–50. [PubMed] [Google Scholar]
- 2.Massy J, Gruber D, Muraine M, et al. La sclerectomie profonde non perforante dans le traitement chirurgical du glaucome chronique a angle ouvert. J Fr Ophtalmol 1999;22:292–8. [PubMed] [Google Scholar]
- 3.Dahan E, Drüsedau MU. Nonpenetrating filtration surgery for glaucoma: control by surgery only. J Cataract Refract Surg 2000;26:695–701. [DOI] [PubMed] [Google Scholar]
- 4.Carassa RG, Bettin P, Fiori M. Viscocanalostomy: a pilot study. Eur J Ophthalmol 1998;8:57–61. [DOI] [PubMed] [Google Scholar]
- 5.Drüsedau MU, von Wolff K, Bull H, et al. Viscocanalostomy for primary open-angle glaucoma: The Gross Pankow experience. J Cataract Refract Surg 2000;26:1367–73. [DOI] [PubMed] [Google Scholar]
- 6.Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg 1999;25:316–22. [DOI] [PubMed] [Google Scholar]
- 7.Lüke C, Dietlein TS, Jacobi PC, et al. A prospective randomized trial of viscocanalostomy versus trabeculectomy in open-angle glaucoma—a one-year follow-up. J Glaucoma 2002;(in press). [DOI] [PubMed]
- 8.Fyodorov SN, Kozlov VI, Timoshkina NT. Non-penetrating deep sclerectomy in open-angle glaucoma. IRTC Eye Microsurg 1989:52–5.
- 9.Kozlov VI, Bagrov SN, Anisimova SY. Non-penetrating deep sclerectomy with collagen. IRTC Eye Microsurg 1989:44–6.
- 10.Sanchez E, Schnyder CC, Sickenberg M, et al. Deep sclerectomy: results with and without collagen implant. Int Ophthalmol 1997;20:157–62. [DOI] [PubMed] [Google Scholar]
- 11.Sourdille P, Santiago PY, Villain F, et al. Reticulated hyaluronic acid implant in nonperforating trabecular surgery. J Cataract Refract Surg 1999;25:332–9. [DOI] [PubMed] [Google Scholar]
- 12.El Sayyad F, Helal M, El-Kholify H, et al. Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology. 2000;107:1671–4. [DOI] [PubMed] [Google Scholar]
- 13.Jonescu-Cuypers C, Jacobi PC, Konen W, et al. Primary viscocanalostomy versus trabeculectomy in white patients with open-angle glaucoma: a randomized clinical trial. Ophthalmology 2001;108:254–8. [DOI] [PubMed] [Google Scholar]
- 14.Demailly P, Lavat P, Kretz G, et al. Non-penetrating deep sclerectomy (NPDS) with or without collagen device (CD) in primary open-angle glaucoma: middle-term retrospective study. Int Ophthalmol 1996. –7;20:131–40. [DOI] [PubMed] [Google Scholar]
- 15.Marchini G, Marraffa M, Brunelli C, et al. Ultrasound biomicroscopy and intraocular-pressure-lowering mechanisms of deep sclerectomy with reticulated hyaluronic acid implant. J Cataract Refract Surg 2001;27:507–17. [DOI] [PubMed] [Google Scholar]
- 16.Spinelli D, Curatola R, Faroni E. Comparison between deep sclerectomy with reticulated hyaluronic acid implant and trabeculectomy in glaucoma surgery. Acta Ophthalmol Scand Suppl 2000;78:60–2. [DOI] [PubMed] [Google Scholar]
- 17.Khaw PT, Siriwardena D. “New” surgical treatments for glaucoma. Br J Ophthalmol 1999;83:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez E, Schnyder CC, Mermoud A. Comparative results of deep sclerectomy transformed in trabeculectomy and those of standard trabeculectomy. Klin Mon Augenheilkd 1997;210:261–4. [DOI] [PubMed] [Google Scholar]
- 19.Lüke C, Dietlein TS, Jacobi PC, et al. Intracorneal inclusion of high-molecular-weight sodium hyaluronate following detachment of Descemet’s membrane during viscocanalostomy. Cornea 2000;19:556–7. [DOI] [PubMed] [Google Scholar]
- 20.Unlu K, Aksunger A. Descemet membrane detachment after viscocanalostomy. Am J Ophthalmol 2000;130:833–4. [DOI] [PubMed] [Google Scholar]
- 21.Mermoud A. Sinusotomy and deep sclerectomy. Eye 2000;14:531–5. [DOI] [PubMed] [Google Scholar]
- 22.Smit BA, Johnstone MA. Effects of viscocanalostomy on the histology of Schlemm’s canal in primate eyes. Invest Ophthalmol Vis Sci 2000;41:S578. [Google Scholar]
- 23.Sit AJ, Coloma FM, Ethier CR, et al. Factors affecting the pores of the inner wall endothelium of Schlemm’s canal. Invest Ophthalmol Vis Sci 1997;38:1517–25. [PubMed] [Google Scholar]
- 24.Johnson DH, Johnson M. How does nonpenetrating glaucoma surgery work? Aqueous outflow resistance and glaucoma surgery. J Glaucoma 2001;10:55–67. [DOI] [PubMed] [Google Scholar]
- 25.Mermoud A, Karlen ME, Schnyder CC, et al. Nd:Yag goniopuncture after deep sclerectomy with collagen implant. Ophthalmic Surg Lasers 1999;30:120–5. [PubMed] [Google Scholar]