Abstract
Background/aim: Proliferative vitreoretinopathy (PVR) and macular pucker (MP) vitreoretinal membranes are caused by abnormal cell migration. By their role in chemotactism, chemokine receptors represent good candidates to sustain this process. The authors thus investigated the expression of one of them, CXCR4, in these pathologies.
Methods: Three PVR and four MP membranes were surgically removed and processed for immunochemical studies with antibodies for CXCR4, cytokeratins or smooth muscle actin.
Results: CXCR4 expression was found in all membranes. There was no relation between severity of PVR or MP and presence of CXCR4. In addition, there was no difference in CXCR4 expression between MP and PVR.
Conclusion: CXCR4 is expressed in PVR and MP. Further experiments are needed to test if CXCR4 and other chemokine receptors are implicated in vitreoretinal membrane formation.
Keywords: vitreoretinal membranes, RPE cells, CXCR4, chemokine receptor
Proliferative vitreoretinopathy (PVR) and macular pucker (MP) represent two forms of vitreoretinal membranes (VRM).1 Both are characterised by the formation of contractile membranes caused by an abnormal migration and proliferation of retinal pigment epithelial cells (RPE), together with other cell types (glial cells, fibroblasts, and inflammatory cells). In PVR, this process is usually important, with membrane proliferation into the vitreous causing, by its contraction, further retinal detachment. In macular pucker, the process is self limited to the macula and will not lead to retinal detachment, but can however impair vision.
The physiopathology of both VRM is characterised by three main aspects: an abnormal migration of cells, followed by a proliferation phase, in an inflammatory context. Interestingly, data accumulated to illustrate the proliferative and inflammatory components of VRM, focusing on a central role of RPE cells.2–4 Nevertheless, the mechanisms of abnormal migration of RPE and other cells are still unclear. By having major roles in chemotactism, chemokines and their receptors represent candidates of choice to sustain this abnormal migration.
Chemokine receptors mediate signalling of chemokines, a superfamily of cytokines implicated in cell migration as well as inflammation, wound healing, and tissue homeostasis.5 If the biology of chemokines has been well studied in PVR, few data are available regarding their receptors.6–8 Chemokine receptor expression is usually restricted to cells of the haematopoietic lineage, in contrast with their ligands, expressed by many different cell types. An important exception to this rule is CXCR4, a chemokine receptor expressed on glial, endothelial, and epithelial cells.9 Interestingly, Crane et al have described that, in vitro, RPE cells functionally expressed CXCR4.10
Considering both the importance of chemokine receptors in mediating cell migration and the central part played by RPE cells in VRM development, we investigated CXCR4 expression in VRM.
MATERIALS AND METHODS
A total of seven VRM were analysed: four MP and three PVR. All MP were idiopathic and all PVR were secondary to retinal detachment surgery. In all cases, surgery consisted of a pars plana vitrectomy with peeling of the membrane from the retina and extraction of the tissue from the vitreous. All specimens were then fixed in 10% neutral buffered formalin, routinely processed, and embedded in paraffin wax. Immunohistochemical studies were performed on 4 μm thick paraffin sections using a Ventana Nexes Staining System (Ventana Medical Systems, Tuscon, AZ, USA), and a DAB detection kit provided by the manufacturer (Ventana). Antibodies directed against the following antigens were used: CXCR4 (clone 12G5, dilution 1:50, Pharmingen, BD Bioscience, Heidelberg, Germany), cytokeratins AE1/AE3 (Dako, A/S, Glostrup, DK), smooth muscle actin SMA (clone 1A4, prediluted), as well as an isotypic control (clone G155–178, Pharmingen, dilution 1:50). Briefly, after 30 minutes of incubation at 37°C with primary antibody, sections were incubated for 10 minutes at 37°C with secondary biotinylated antibody, then with avidin-peroxidase for the same time; 3’,3-diaminobenzidine ( DAB) was used as chromogen. Slides were counterstained with haematoxylin and eosin, dehydrated, and mounted before observation under an optical microscope. Positive cells were identified by a brown stain. Slides were carefully analysed by two independent pathologists.
RESULTS
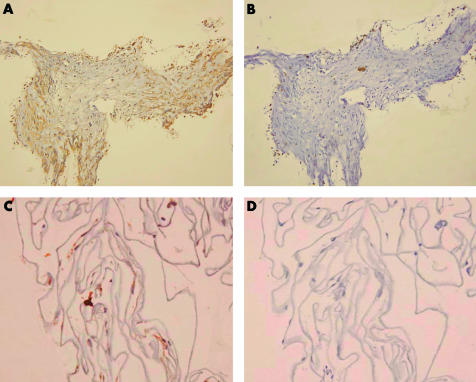
CXCR4 expression was found in all VRM (Fig 1 and Table 1). There was no relation between the severity of PVR or MP and the presence of CXCR4. In addition, there was no difference in CXCR4 expression observed between MP and PVR (Table 1). Results from cell characterisations are summarised in Table 1. Haematoxylin and eosin sections revealed that PVR specimens were heterogeneous with the presence of fusiform cells, pigmented cells, and inflammatory infiltrates. This cell diversity was reflected by immunostaining: one PVR specimen was positive for SMA, one was positive for cytokeratins AE1/AE3, and one was positive for both. In contrast, MP were homogeneous, composed of elongated fusiform cells and few pigmented cells. Accordingly, all MP specimens were positive for SMA and negative for cytokeratins AE1/AE3. CXCR4 positivity was observed in all cell types.
Figure 1.
(A) Proliferative vitreoretinopathy: CXCR4 staining. (B) Proliferative vitreoretinopathy: negative isotypic control. (C) Macular pucker: CXCR4 staining. (D) Macular pucker: negative control. Magnification: ×200.
Table 1.
Summary of VRM immunohistochemical stainings
| CXCR4 | SMA | CK | HE | |
| 1 PVR | + | – | + | Pigmented cells |
| 2 PVR | + | + | + | Pigmented cells |
| Fusiform cells | ||||
| Inflammatory cells | ||||
| 3 PVR | + | + | – | Pigmented cells |
| Fusiform cells | ||||
| Inflammatory cells | ||||
| 4 MP | + | + | – | Pigmented cells |
| Fusiform cells | ||||
| 5 MP | + | + | – | Pigmented cells |
| Fusiform cells | ||||
| 6 MP | + | + | – | Pigmented cells |
| Fusiform cells | ||||
| 7 MP | + | ND | ND | Pigmented cells |
| Fusiform cells |
HE = haematoxylin and eosin, CK = cytokeratin, SMA = smooth muscle actin, ND = not done.
DISCUSSION
One of the most important steps of VRM formation is abnormal cell migration. Chemokines and chemokine receptors are specialised in cell migration control. Chemokine receptors expression allows cells to migrate in response to a gradient of chemokine ligands.
If the implication of chemokines in VRM has been highlighted by others, to our knowledge none has yet reported on the potential role of chemokine receptors.6–8 Here we show that CXCR4 is expressed in pathological specimens of VRM (PVR and MP).
CXCR4 and its ligand SDF-1 differ from other chemokine-ligand pairs. Firstly, CXCR4 is one of the very few chemokine receptors for which only a single ligand has been identified so far. Secondly, CXCR4 and SDF-1 are expressed constitutively in a wide range of tissues including brain, thymus, lymph nodes, spleen, stomach, kidney, and gut.9 A functional in vitro expression of CXCR4 by RPE cells was also recently demonstrated by Crane et al.11 They indeed showed that RPE cells expressed both CXCR4 and SDF-1, and that in vitro SDF-1 stimulation resulted in RPE cell migration Accordingly, we also observed that CXCR4 is expressed in enucleated eyes, on hRPE cells (data not shown).
Theoretically, thus, if SDF-1 is produced in vivo, RPE cells should migrate in response to its gradient, as should glial cells, another important cell type in VRM. We did not look for SDF-1 secretion in the vitreous of our patients. But a similar role has been attributed to the CXCR4-SDF-1 pair in the resolution phase of wound healing, a pluricellular process which in many ways mimics VRM formation.11 In addition, other chemokines (that is, MCP-1) were detected in the vitreous of patients with VRM, suggesting that they might be of importance, as their respective chemokine receptors, in VRM cell recruitment.6–8 In conclusion, we showed that CXCR4 is expressed in VRM, but further experiments are needed to define the role of CXCR4 and other chemokine receptors in VRM formation.
Acknowledgments
This work has been supported by a grant from the Fonds de Recherche en Ophtalmologic, Fondation Vesale, and the Fondation Horlait-Dapsens.
L Cabay and F Willermain have contributed equally to this work.
REFERENCES
- 1.Charteris D. Proliferative vitreoretinopathy pathobiology, surgical management, and adjunctive treatment. Br J Ophthalmol 1995;79:953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morino I, Hiscott P, McKechnie N, et al. Variation in epiretinal membrane components with clinical duration of the proliferative tissue. Br J Ophthalmol 1990;74:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shinoda K, Hirakata A, Hida T, et al. Ultrastructural and immunohistochemical findings in five patients with vitreomacular traction syndrome. Retina 2000;20:289–93. [DOI] [PubMed] [Google Scholar]
- 4.Abe T, Durlu YK, Tamai M. Dedifferentiation of the retinal pigment epithelium compared to the proliferative membranes of proliferative vitreoretinopathy. Curr Eye Res 1998;17:1103–9. [DOI] [PubMed] [Google Scholar]
- 5.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol 2000;18:217–42. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed M, El-Asrar A. Monocyte chemotactic protein-1 in proliferative vitreoretinal disorders. Am J Ophthalmol 1997;123:599–606. [DOI] [PubMed] [Google Scholar]
- 7.El-Ghably IA, Dua HS, Orr GM. Detection of cytokine mRNA production in infiltrating cells in proliferative vitreoretinal using reverse transcription polymerase chain reaction. Br J Ophthalmol 1999;83:1296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capeans C, De Rojas MV, Lojo S. C-C chemokines in the vitreous of patients with proliferative vitreoretinopathy and proliferative diabetic retinopathy. Retina 1998;18:546–50. [PubMed] [Google Scholar]
- 9.Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev 2000;177:175–84. [DOI] [PubMed] [Google Scholar]
- 10.Crane I, Wallace C, McKillop-Smith, et al. CXCR4 receptor expression on human retinal pigment epithelial cells from the blood-retina barrier leads to chemokine secretion and migration in response to stromal cell-derived factor 1. J Immunol 2000;165:4372–8. [DOI] [PubMed] [Google Scholar]
- 11.Fedyk E, Jones D, Critchley H, et al. Expression of stromal-derived factor-1 is decreased by IL-1 and TNF in dermal wound healing. J Immunol 2001;166:5749–54. [DOI] [PubMed] [Google Scholar]