Abstract
The downstream box (DB) is a sequence element that enhances translation of several bacterial and phage mRNAs. It has been proposed that the DB enhances translation by base pairing transiently to bases 1469–1483 of 16S rRNA, the so-called anti-DB, during the initiation phase of translation. We have tested this model of enhancer action by constructing mutations in the anti-DB that alter its mRNA base-pairing potential and examining expression of a variety of DB-containing mRNAs in strains expressing the mutant anti-DB 16S rRNA. We found that the rRNA mutant was viable and that expression of all tested DB-containing mRNAs was completely unaffected by radical alterations in the proposed anti-DB. These findings lead us to conclude that enhancement of translation by the DB does not involve mRNA–rRNA base pairing.
Initiation of translation in bacteria typically involves two base-pairing interactions: the mRNA initiation codon pairs with the anticodon of the initiator methionine tRNA, and the Shine–Delgarno (SD) sequence, 7–10 bases upstream of the initiation codon, pairs with the 3′ end of 16S rRNA (1). Mutagenesis studies of the initiation signals of particular mRNAs as well as analysis of atypical and leaderless mRNAs have identified other sequence elements surrounding the initiator AUG that contribute to the efficiency of the initiation signal (2). One such class of such elements has been termed translational enhancers, based on their ability to enhance the expression of various mRNA reporter gene constructs or to promote initiation in the absence of any identifiable SD. Among the best-studied enhancer elements is the downstream box (DB), so called because of its location downstream of the AUG initiation codon (3, 4). This sequence was first identified by mutagenesis studies in the mRNA of phage T7 gene 0.3 and subsequently found in the λcI, lysU, glnS, rpoH, and cspA mRNAs of Escherichia coli (5–10). Similar elements have been found in the dnaX gene of Caulobacter crescentus, the vph gene of Streptomyces vinaceus, and the rnhB gene of Streptococcus pneumoniae (11–13). All of the identified DB elements display partial complementarity to nucleotides 1469–1483 of 16S rRNA. Moreover, mutagenesis studies have indicated that, in general, increases in the level of complementarity to this region of 16S rRNA led to increased expression whereas mutations that decreased complementarity caused corresponding reductions in expression of the reporter genes. Based on these observation, it has been concluded that DB elements enhance and stabilize the interactions of initiating ribosomes with mRNAs by base pairing to nucleotides 1469–1483 of 16S rRNA, the so-called anti-DB.
Although the mRNA mutagenesis studies cited above lend considerable credence to the mRNA–rRNA base-pairing model of enhancer action, supporting biochemical or rRNA mutagenesis data are lacking. Base-pairing interactions between mRNA and rRNA place considerable constraints on the orientation of mRNA within the ribosome. The mRNA path through the ribosome has been studied extensively by mRNA crosslinking studies (14). However, to date, no crosslinks involving nucleotides 1469–1483 have been identified (R. Brimacombe, personal communication). In a study of ribosome-mediated protection of mRNA from base modification by chemical probes, Huttenhofer and Noller (15) failed to observe any protection of the DB region T4 gene 32 mRNA. More recently, Resch et al. (16) have shown that the proposed DB–rRNA interaction is dispensable for initiation of leaderless mRNAs, including the λcI mRNA. Because of the widespread distribution of DB elements and their proposed importance in the initiation process, we have tested the base-pairing model by constructing mutations in the anti-DB of 16S rRNA and examining the effects of the 16S rRNA mutations on the expression of DB-containing mRNAs. Our findings are that mutations in the proposed anti-DB had little effect on cell growth/viability and had no effect on the expression of a variety of DB-containing reporter gene constructs. We conclude, therefore, that although DB elements may contribute to the efficiency of initiation, the mechanism of enhancement of translation does not involve mRNA–rRNA base pairing.
MATERIALS AND METHODS
Bacterial Strains and Plasmids.
Plasmids pKK3535 and pMO10 each contain an intact wild-type rrnB operon and were used as rRNA expression vectors (17). Plasmid pKK1192U is derived from pKK3535 and contains the C1192U spectinomycin resistance mutation in 16S rRNA. Plasmids pMO28 and pMO31 are derived from plasmid pACYC177; pMO28 contains an intact rrnB operon and the C1192U spectinomycin resistance mutation whereas pMO31 contains both the C1192U and anti-DB Flip rRNA mutations. Addition of spectinomycin to cultures of strains expressing these rRNAs ensures that only plasmid-encoded, spectinomycin-resistant ribosomes are active in translation. Strain TA548 is ΔrrnE ΔrrnB ΔrrnA ΔrrnH ΔrrnG∷cat ΔrrnC∷cat ΔrrnD∷cat recA56/pTRNA66, pSTL102 (18) and was used as a host for pMO10-derived rrnB plasmids, pBR322-derived lacZ plasmids, and plasmids expressing the vph viomycin resistance gene. Strain TA531 (ΔrrnE ΔrrnB ΔrrnA ΔrrnH ΔrrnG∷lacZ ΔrrnC∷cat ΔrrnD∷cat ΔrecA/pTRNA66 pHKrrnC) was used as a host for pKK3535 and its mutant derivatives. The ampicillin-resistant, rrnB-containing plasmid, pSTL102, in strain TA548 was replaced with neomycin-resistant, pMO10-derived plasmids by transforming TA548 with the neomycin-resistant plasmids and growing the transformants in the absence of selection for pSTL102. Loss of pSTL102 was monitored by the appearance of ampicillin-sensitive clones after 3–4 cycles of overnight growth and dilution into fresh medium in the absence of ampicillin selection. A similar strategy was used to replace the neomycin-resistant plasmid pHKrrnC with the ampicillin-resistant plasmid pKK3535 in strain TA531. Strain MC1061 (F− araD139 Δ(ara-leu)7696 galE15 galK16 ΔlacX74 rpsL hsdR2 mcrA mcrB1) was used as a host for pMC1871-derived lacZ constructs.
The ampicillin-resistant lysU-lacZ fusion plasmids pRP92, pRP103, and pRP105 were obtained from Yoshikazu Nakamura, University of Tokyo (7). The λcI-lacZ fusion plasmids pdb and pdb* were obtained from Max Gottesman, Columbia University (6). The glnS-lacZ fusion plasmids pJP34 ad pJP35 were obtained from Leif Isaksson, Stockholm University (8). The wild-type (λGF34 and λGF364) and mutant (λGF364-D1) rpoH-lacZ fusions were obtained as MC4100 lysogens, and a further mutant rpoH-lacZ fusion (pFRP103–15A) was obtained as a low-copy plasmid from Takashi Yura, HSP Research Institute, Kyoto, Japan (9). MC4100 lysogens were transformed with pKK1192U or pKK1192U-mDB, encoding spectinomycin-resistant, wild-type, and the anti-DB-Flip 16S rRNAs, respectively. β-Galactosidase levels were measured in cultures of these lysogenic strains grown in the presence of spectinomycin at 30°C. TA548-derived strains containing wild-type or mutant pMO10 plasmids were transformed with the various λcI-/lysU-/rpoH-lacZ fusion plasmids, and β-galactosidase was measured in cultures grown in minimal medium at 30°C as described previously (19). Because of unanticipated problems with instability of the glnS-lacZ plasmids in strain TA548, the effects of rRNA mutations on expression of glnS-lacZ fusions were investigated in strain TA430 (ΔrrnE ΔrrnB ΔrrnH ΔrrnA∷cat) carrying deletions in four rrn operons. TA430 first was transformed with each of the glnS-lacZ fusion plasmids and subsequently with pMO28 or pMO31 expressing spectinomycin-resistant, wild-type, and the anti-DB-Flip 16S rRNAs, respectively. β-Galactosidase levels then were measured in cultures of these strains grown in the presence of spectinomycin, neomycin, and ampicillin at 30°C.
The viomycin resistance plasmids, pIU455 and pIU456, together with a vector control plasmid, pIU433, were obtained from Gary Janssen, Miami University, Oxford, Ohio (12). TA548 derivatives containing either wild-type or mutant pMO10 rrnB plasmids were transformed with each of the vph plasmids, or the vector control, and expression of the viomycin resistance gene was monitored by growth of the strains in increasing concentrations of viomycin (0, 100, 200, and 300 mg/liter) at 30°C, as described previously (12).
Plasmid pMC1871 contains a promoterless lacZ gene and was used for the construction of mutant derivatives of the wild-type rpoH-lacZ-fusion (20). Growth rates of TA548 and TA531 derivatives containing pMO10 or pKK3535 derivatives, respectively, were measured by diluting overnight cultures of these strains into fresh LB and following the increases in turbidity thereafter by using a Klett–Summerson colorimeter.
Mutagenesis.
Site-directed mutagenesis of rRNA was carried out on an M13 mp18 EcoRI-XbaI clone carrying the 3′ end of 16S rRNA, as described by Kunkel (21). Mutant M13 clones were identified by DNA sequencing, and mutant derivatives of plasmids pMO10, pKK3535, and pKK1192U were constructed by replacing the wild-type BglII-XbaI fragment in the plasmid with the mutant fragment from the M13 clone.
Mutagenesis of rpoH-lacZ fusions was carried out by the megaprimer PCR method as described by Barik (22). In the first PCR, primers incorporating the desired mutation (rpoHDB+1, ATGACTGGACAAAATACAAAGTTTGCTTTAGCC; RPOHDB+2, ATGACTGAGACAAAATACAAAGTTGCTTTAGCC; or rpoHDB+3, ATGACTGGAGACAAAATACAAAGTGCTTTAGCC) together with primer lacZSacI (GCGCCACCATCCAGTGCAGGAGCTCGTTATCGC) encompassing the unique SacI site in lacZ were used as forward and reverse primers, respectively, and plasmid pFRP103–15A was used as template. In the second PCR, the primer rpoH3Xma (GCGATTGTCATCCCGGGTTGCGGAAGTGGCACAGGTTTTCGG) that annealed to a region 374 bases upstream of the rpoH AUG start codon and incorporated nucleotide changes generating a unique XmaI restriction site was used as the forward primer, and the PCR product from the first PCR was used as the reverse primer. The products of the second PCR were cleaved with XmaI and SacI and annealed to XmaI-SacI-cleaved plasmid pMC1871. The resulting mutant plasmids were verified by nucleotide sequencing. The wild-type and mutant rpoH-lacZ fusions carried on the λGF364 prophage and pFRP103–15A, respectively, also were transferred to the lacZ fusion plasmid pMC1871. This was achieved by using primers rpoH3Xma and lacZSacI to amplify rpoH-lacZ fragments from the fusion-containing strains, treating the products of these PCRs with XmaI and SacI and ligating the DNAs to XmaI-SacI-cleaved plasmid pMC1871. Transformations, DNA extractions, and other standard procedures were carried out as described (23).
RESULTS
Construction and Expression of Mutations in the Anti-DB of 16S rRNA.
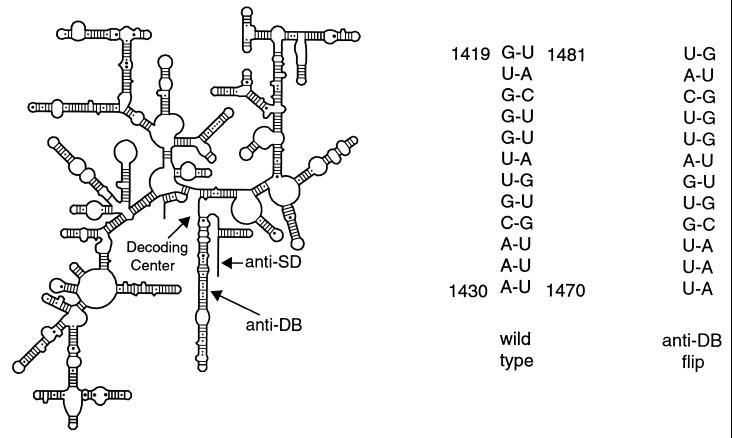
Nucleotides 1470–1481 of 16S rRNA form part of a conserved helical structure and are paired with nucleotides 1419–1430 (Fig. 1). Our previous work on this region of 16S rRNA indicates that mutations on either side of the helix that disrupt base pairing affect subunit–subunit interactions, decrease the fidelity of translation, and increase the doubling times of cells expressing these mutant rRNAs (24). These effects on ribosome function are virtually eliminated when base pairing within the helix is restored through the introduction of compensatory base pairs. It thus appears that a stable base-paired helix in this region of rRNA, rather than a particular sequence per se, is important for proper ribosome function. Based on these findings, we have constructed a multiple mutation (anti-DB flip mutant; Fig. 1) that reversed all 12 bp within this helical region of 16S rRNA and expressed the mutant rRNA on a plasmid copy of the rrnB operon, from the constitutive P1P2 promoters. It was hoped that by creating a mutant helix of the same base composition and stability as wild-type 16S rRNA, any deleterious effects on subunit association and decoding would be avoided. However, an important consequence of reversing the base pairs in this region of rRNA was that the polarity of the proposed anti-DB sequence was reversed in the mutant rRNA and, consequently, its mRNA base-pairing potential was radically altered.
Figure 1.
Secondary structure of E. coli 16S rRNA with the 1419–1481 regions of wild-type and anti-DB flip rRNA mutants enlarged on the right.
The development of a strain of E. coli in which all of the cellular rRNA is derived from a plasmid-encoded rrn operon has made it possible to analyze the effects of rRNA mutations in the absence of wild-type, chromosomally encoded rRNAs (18). The resident rrn-containing plasmid in this strain can be displaced by another rrn-containing plasmid that carries a different vector-associated, antibiotic resistance marker. Using this approach, we have managed to construct isogenic strains of E. coli that express either wild-type or anti-DB flip mutant rRNAs exclusively. A variety of previously studied, single-base alterations in helix 44 have been found to alter the ability of the mutant subunits to associate with 50S subunits (24–26). Although a very slight increase in the amount of free subunits was observed in sucrose gradients of anti-DB mutant ribosomes, cell lysates from strains expressing exclusively wild-type or the anti-DB flip mutant rRNAs displayed essentially identical levels of 70S ribosomes and polysomes on sucrose gradients. However, the growth rates of strains expressing only plasmid-encoded anti-DB mutant rRNAs were 15% slower than the corresponding wild-type strains; TA531 carrying wild-type or the anti-DB mutant version of pKK3535 had doubling times of 61 ± 1 min and 69 ± 2 min, respectively, whereas strain TA548 carrying wild-type or the anti-DB mutant version of pMO10 had doubling times of 70 ± 3 min and 81 ± 6 min, respectively. The longer doubling times of strains expressing total cellular rRNA from pMO10-based plasmids compared with strains expressing rRNA from pKK3535-derived plasmids is perhaps a result of the lower copy number of the pSC101-derived plasmid, pMO10. The anti-DB mutant ribosomes also stimulated stop codon read-through and frame-shifting at 1.3–1.8 times higher than wild-type ribosomes (data not shown). An effect of mutations in the 1469–1483 region on decoding fidelity is consistent with previous observations that mutations at positions A1431, C1469, and A1483 decreased the accuracy of translation (24, 27, 28). Together, these data indicate that although the sequence of the proposed anti-DB can be altered without having dramatic effects on cell physiology, alterations in the primary sequence of the 1419–1481 region of helix 44 impinge on subunit–subunit interactions and the fidelity of the decoding process.
Effects of Mutations in the Proposed Anti-DB of 16S rRNA on Expression of DB-Containing mRNAs.
The importance of the DB has been investigated in several laboratories by analyzing the effects of mutations in DB sequences on expression of lacZ fusions to the reporter gene of interest. We, therefore, have obtained several of these lacZ constructs and examined the effects of mutations in the anti-DB of 16S rRNA on lacZ expression.
Transcription of phage λcI repressor from the pM promoter generates a transcript that initiates with the A of the AUG codon and, thus, lacks any 5′ leader or SD sequence. Genetic analyses by Shean and Gottesmann (6) identified a DB near the 5′ end of the λcI coding sequence. Mutations within this DB that eliminated 4 bases of complementarity to 16S rRNA reduce expression of a λcI-lacZ fusion 9- to 12-fold. β-Galactosidase assays of cells expressing either wild-type or the anti-DB flip mutant 16S rRNAs showed that although there was a 10-fold difference in β-galactosidase activity between the wild-type (λcI db) and mutant versions (λcI db*) of the λcI-lacZ fusion in both strains, this difference was unaffected by the sequence of the anti-DB of 16S rRNA (Table 1).
Table 1.
Effects of rRNA mutations on expression of DB-containing λcI, lysU, rpoH, and glnSp-lacZ fusions
| lacZ fusion | Plasmid/prophage | Units of β-galactosidase
|
|
|---|---|---|---|
| Wild-type rRNA | Anti-DB flip rRNA | ||
| λcI | pdb | 186 ± 50 | 189 ± 12 |
| pdb* | 15 ± 2 | 18 ± 1 | |
| lysU | pRP92 | 58 ± 4 | 54 ± 4 |
| pRP103 | 701 ± 55 | 622 ± 67 | |
| pRP105 | 737 ± 171 | 695 ± 109 | |
| rpoH | λGF34 | 467 ± 61 | 406 ± 24 |
| λGF364 | 34 ± 2 | 31 ± 1 | |
| pFRP103–15A | 559 ± 49 | 568 ± 62 | |
| λGF364D1 | 12 ± 4 | 11 ± 1 | |
| glnS | pJP34 | 46 ± 2 | 43 ± 9 |
| pJP35 | 268 ± 35 | 247 ± 47 | |
β-Galactosidase activities are given in Miller units (19); each value represents the mean ± SE of assays from four to six independent cultures.
The E. coli lysU gene encodes one of the two cellular lysyl-tRNA synthetases and can be induced by high temperature or growth on certain metabolites. Genetic analysis of the 5′ end of the lysU coding region has identified a DB-containing sequence that is necessary for its high-level expression (7). Cells expressing either mutant or wild-type 16S rRNA were transformed with lysU-lacZ plasmids containing mutations that had either low (2 bases; pRP92) or high levels of rRNA complementarity (6 and 8 bases; pRP103 and pRP105, respectively) and assayed for β-galactosidase activity. These assays (Table 1) showed that although the DB had a substantial effect on the level of lacZ expression, β-galactosidase activity supported by each lysU-lacZ fusion was unaffected by the sequence of the 1470–1481 region of 16S rRNA.
Induction of the heat-shock response in E. coli involves increased synthesis of a heat-shock-specific σ factor, σ32, encoded by the rpoH gene. Increased synthesis of this σ factor upon temperature shift is due largely to increased translation of rpoH mRNA. Analysis of the rpoH transcript has identified both positive and negative cis-acting elements that are responsible for potentially high-level expression at 30°C and thermal regulation, respectively (7). All of these controlling elements are present in the λGF364 rpoH-lacZ fusion, whereas the element(s) responsible for translational repression at 30°C and heat inducibility have been deleted in the λGF34 fusion (7). The region of rpoH mRNA responsible for potentially high-level expression at 30°C is adjacent to the AUG initiation codon and includes a DB sequence. Mutations in this DB element that increased (pFRP103–15A) or decreased (λGF364D1) complementarity to 16S rRNA nucleotides 1469–1483 relative to the wild-type λGF364 fusion had corresponding effects on the level of expression of rpoH-lacZ fusions at 30°C. Analysis of rpoH-lacZ expression in cells expressing exclusively either mutant or wild-type forms of 16S rRNA again showed that although alterations in the DB element affected rpoH-lacZ expression, these levels of β-galactosidase activity were unaffected by the sequence of nucleotides 1470–1481 of 16S rRNA (Table 1).
A GAG → GAA mutation at codon 3 in the glutaminyl-tRNA synthetase (glnS) gene increases expression of a glnS-lacZ fusion 4-fold (8). The effects of further mutations that increased the apparent complementarity of the 5′ end of the glnS mRNA to the anti-DB suggested that increased expression was correlated with increased base-pairing potential to the anti-DB. Measurement of β-galactosidase activities in strains carrying the wild-type glnS-lacZ fusion on plasmid pJP34, or plasmid pJP35 carrying the GAG → GAA mutation, under conditions in which only plasmid-encoded wild-type or anti-DB flip rRNAs were functional, showed that alterations in the proposed anti-DB sequence did not affect glnS-lacZ expression (Table 1).
Analysis of the viomycin phosphotransferase (vph) gene of S. vinaceus has shown that it can be translated effectively in the absence of any leader sequences, in both Streptomyces and E. coli, and that only the first 16 vph codons are required for efficient initiation on this leaderless mRNA (12). This 16-codon sequence is partially complementary to the anti-DBs of both Streptomyces and E. coli and suggested that DB–anti-DB pairing might contribute to the initiation signal of this leaderless mRNA. Strains expressing either wild-type or the anti-DB flip mutant 16S rRNA were transformed with two different vph plasmids (pIU455 and pIU456) or a vector control (pIU433), and the effects of alterations in 16S rRNA on expression of leaderless vph mRNAs were assayed by examining their growth in viomycin-containing medium as described previously (12). All strains carrying vph plasmids grew equally well in viomycin-containing medium (data not shown), indicating that alterations in the 16S rRNA anti-DB did not affect the translation of vph mRNA.
The published data relating to the five sets of DB-containing mRNA constructs described above constitute the majority of the experimental support for the proposed DB–anti-DB pairing. However, although we have been able to confirm the previously reported effects of mutations in the DB region of the various mRNAs, the expression of each of these lacZ constructs and of the vph gene is consistently unaffected by radical alterations in the proposed base-pairing region of 16S rRNA. We conclude, therefore, that although the DB element may contribute substantially to the efficiency of translational initiation, its effect on translation does not involve mRNA–rRNA base pairing.
Testing the Reading-Frame Dependence of the DB Enhancer.
Analyses of codon distribution in E. coli mRNAs by several groups have reported a nonrandom distribution of codons close to the initiation site (29, 30). Such an arrangement of codons might promote rapid movement of the just-initiated ribosome away from the initiation region, thereby facilitating subsequent initiation events by other ribosomes. We have asked whether the enhancer effect of the DB is related to the choice of codons that constitute this sequence by testing its ability to enhance translation in different reading frames. A single G → A mutation in the DB region of the rpoH mRNA increases expression 5- to 10-fold at 30°C (compare activities supported by plasmid pFRP103–15A with the wild-type λGF364 fusion; Table 1 and ref. 9). For ease of manipulation, both of these fusions were reconstructed in the multicopy lacZ plasmid pMC1871 and designated pDBZGA and pDBZwt, respectively. Using the G→A high-expression mutant as a starting point, one, two, or three base insertions and deletions were made at the 5′ and 3′ sides of the DB element, respectively, by site-directed mutagenesis. In this way, a series of rpoH-lacZ fusions was constructed that contained the DB element in the 0, +1, +2, and +3 reading frames (designated pDBZGA, pDBZ+1, pDBZ+2, and pDBZ+3; Table 2). Activity measurements of these constructs showed that the 0, +1, and +2 reading-frame constructs all had equivalent β-galactosidase activities (ca. 7,000 units in all cases). Surprisingly, the +3 reading-frame construct that contains the same codons comprising the DB as in the 0 frame construct displayed significantly lower activity (ca. 4,000 units). Nevertheless, the finding that the 0, +1, and +2 frame constructs support equivalent levels of β-galactosidase demonstrates that the enhancer activity is not dependent on the codons that constitute the DB and suggests that the enhancer activity is intrinsic to the RNA sequence itself.
Table 2.
Testing the reading-frame dependence of the rpoH enhancer
| Plasmid | Sequence | Activity |
|---|---|---|
| pDBZwt | AUG ACU CAC AAA AUG CAA AGU UUA GCU UUA GCC CCA AGU | 1,448 ± 384 |
| pDBZGA | AUG ACU GAC AAA AUA CAA AGU UUA GCU UUA GCC CCA AGU | 7,688 ± 930 |
| pDBZ+1 | AUG ACU GGA CAA AAU ACA AAG UUU *GCU UUA GCC CCA AGU | 7,558 ± 1,296 |
| pDBZ+2 | AUG ACU GAG ACA AAA UAC AAA GUU **GCU UUA GCC CCA AGU | 5,986 ± 981 |
| pDBZ+3 | AUG ACU GGA GAC AAA AUA CAA AGU ***GCU UUA GCC CCA AGU | 3,937 ± 788 |
Activity measurements are given in Miller units of β-galactosidase activity (19). The G → A substitution that increases the activity of the wild-type rpoH-lacZ fusion 5- to 10-fold is indicated in italics. Base insertions are indicated in bold. Positions of base deletions are indicated by asterisks (∗).
DISCUSSION
The original proposal by Shine and Delgarno (31) that initiation of translation involved base pairing of the 3′ end of 16S rRNA to complementary sequences upstream of the initiation codon on the mRNA was based solely on sequence comparisons of a limited number of bacterial and phage mRNAs. Nevertheless, in the 25 years since this proposal was first advanced, the rRNA–mRNA base-pairing model for initiation has been supported by numerous biochemical and genetic experiments, as well as by comparative sequence analyses (32–35). Chemical footprinting of DB-containing mRNAs, using probes that are sensitive to base-pairing interactions, has been used to examine the putative interaction of the DB with rRNA (15, 16). These studies have shown that although the SD region of the mRNA was protected by ribosomes from chemical modification in a manner consistent with rRNA–mRNA base pairing, the DB region was not protected. In this study, we have examined the proposed base pairing of the DB to 16S rRNA by manipulation of rRNA sequences by using the same approach that we and others have used previously to examine the SD–anti-SD interaction (33–35). We find that expression of each DB-containing mRNA tested is completely unaffected by radical alterations in the proposed anti-DB sequence in rRNA, indicating that base pairing is not involved in enhancement of translation of these mRNAs. The results of our mutagenesis experiments lead us to conclude that the DB does not engage in base pairing with the proposed anti-DB, whereas the chemical footprinting experiments indicate that the DB does not pair with this or any other region of rRNA. We conclude, therefore, that the proposed anti-DB does not exist.
The evidence in support of the base-pairing model derives wholly from a series of limited alterations of DB-containing mRNAs and the observation that, in general, mutations that increased or decreased complementarity to the proposed anti-DB led to corresponding changes in gene expression. As can be seen in Table 1, the effects of these altered DBs on expression are maintained in strains expressing either mutant or wild-type rRNA. Thus, although the DB does not engage in base pairing with rRNA, the presence and primary structure of this element does affect gene expression, and other explanations must be sought to account for its ability to enhance translation. Moreover, because the DB–anti-DB base-pairing model is no longer tenable, it is unclear that all of the existing DBs enhance translation by the same mechanism(s), and, consequently, extensive mutagenesis studies are now required to identify and define the sequence requirements of DB elements.
The results of mutagenesis of DB-containing mRNAs that previously had been used to support the base-pairing model are equally consistent with a number of other explanations. It has been proposed that the increase in glnS expression caused by a single base mutation in the glnS DB might be due to the substitution of a rapidly translated GAA codon for the slower-translated GAG codon (8, 36). Although as we show in Table 2, the choice of codons constituting the rpoH DB does not appear to affect its expression, this result does not preclude a possible effect of codon choice on the activity of other DBs. In addition, the possible effects of DB mutations on RNA structure and mRNA or protein stability have not been investigated fully and might contribute in part or in full to the enhancing effects of some DBs (37–39).
In addition to the DB, a number of other non-SD-related initiation elements have been shown to promote or enhance translation in E. coli (40–42). Although various rRNA–mRNA base-pairing schemes have been advanced to explain these enhancer effects, it also has been suggested that at least some of these pyrimidine-rich sequences promote efficient initiation by interactions with ribosomal protein S1, and the omega element from tobacco mosaic virus RNA that promotes translation in the absence of a SD element has been shown to bind S1 directly in vitro (43, 44). More recently, Ringquist et al. (44) have shown that S1 recognizes highly structured RNAs, and, consequently, the RNA sequences recognized by ribosomal protein S1 may be more extensive than previously had been appreciated. Therefore, the possibility that some of the DB sequences enhance translation by interacting with S1 cannot be excluded and currently is being tested.
Although base-pairing models have provided an attractive basis for contemplating RNA–rRNA interactions, of all such models proposed, only the SD–anti-SD interaction and the base pairing of C74 at the 3′ end of all tRNAs with G2252 in 23S rRNA have withstood rigorous analysis (45). It appears, therefore, that mRNA–rRNA interactions proposed on the basis of base complementarity alone, in the absence of supporting genetic and biochemical data, should be approached with caution.
Acknowledgments
We are indebted to Drs. Magnus Stenstrom, Leif Isaksson, Max Gottesman, Gary Janssen, Yoshikazu Nakamura, and Takashi Yura for supplying us with the various DB-containing lacZ fusions that made this study possible. We are grateful to Dr. George Q. Pennabble for his insights. This work was supported by Grants GMS19756 (to A.E.D.) and GM24751 (to C.L.S.) from the National Institutes of Health.
ABBREVIATION
- DB
downstream box
References
- 1.Gualerzi C O, Pon C L. Biochemistry. 1990;29:5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- 2.Ringquist S, Shinedling S, Barrick D, Green L, Binkley J, Stormo G D, Gold L. Mol Microbiol. 1992;6:1219–1229. doi: 10.1111/j.1365-2958.1992.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 3.Sprengart M L, Fuchs E, Porter A G. EMBO J. 1996;15:665–674. [PMC free article] [PubMed] [Google Scholar]
- 4.Sprengart M L, Porter A G. Mol Microbiol. 1997;24:19–28. doi: 10.1046/j.1365-2958.1997.3161684.x. [DOI] [PubMed] [Google Scholar]
- 5.Sprengart M L, Fatscher H P, Fuchs E. Nucleic Acids Res. 1990;18:1719–1723. doi: 10.1093/nar/18.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shean C S, Gottesman M E. Cell. 1992;70:513–522. doi: 10.1016/0092-8674(92)90175-c. [DOI] [PubMed] [Google Scholar]
- 7.Ito K, Kawakami K, Nakamura Y. Proc Natl Acad Sci USA. 1993;90:302–306. doi: 10.1073/pnas.90.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faxen M, Plumbridge J, Isaksson L A. Nucleic Acids Res. 1991;19:5247–5251. doi: 10.1093/nar/19.19.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagai H, Yuzawa H, Yura T. Proc Natl Acad Sci USA. 1991;88:10515–10519. doi: 10.1073/pnas.88.23.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitta M, Fang L, Inouye M. Mol Microbiol. 1997;26:321–335. doi: 10.1046/j.1365-2958.1997.5771943.x. [DOI] [PubMed] [Google Scholar]
- 11.Winzeler E, Shapiro L. J Bacteriol. 1997;179:3981–3988. doi: 10.1128/jb.179.12.3981-3988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C J, Janssen G R. Mol Microbiol. 1996;22:339–355. doi: 10.1046/j.1365-2958.1996.00119.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y B, Ayalew S, Lacks S. J Bacteriol. 1997;179:3828–3836. doi: 10.1128/jb.179.12.3828-3836.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sergiev P V, Lavrik I N, Wlasoff V A, Dokudovskaya S S, Dontsova O A, Bogdanov A A, Brimacombe R. RNA. 1997;3:464–475. [PMC free article] [PubMed] [Google Scholar]
- 15.Huttenhofer A, Noller H F. EMBO J. 1994;13:3892–3901. doi: 10.1002/j.1460-2075.1994.tb06700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resch A, Tedin K, Grundling A, Mundlein A, Blasi U. EMBO J. 1996;15:4740–4748. [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory S T, Brunelli C A, Lodmell J S, O’Connor M, Dahlberg A E. Methods Mol Biol. 1998;77:271–281. doi: 10.1385/0-89603-397-X:271. [DOI] [PubMed] [Google Scholar]
- 18.Asai T, Zaporojets D, Squires C, Squires C L. Proc Natl Acad Sci USA. 1999;96:1971–1976. doi: 10.1073/pnas.96.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor M, Goringer H U, Dahlberg A E. Nucleic Acids Res. 1992;20:4221–4227. doi: 10.1093/nar/20.16.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapira S K, Chou J, Richaud F V, Casadaban M J. Gene. 1983;25:71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- 21.Kunkel T A. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barik S. Methods Mol Biol. 1996;57:203–215. doi: 10.1385/0-89603-332-5:203. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 24.Firpo M A, Dahlberg A E. Nucleic Acids Res. 1998;26:2156–2160. doi: 10.1093/nar/26.9.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rottmann N, Kleuvers B, Atmadja J, Wagner R. Eur J Biochem. 1988;177:81–90. doi: 10.1111/j.1432-1033.1988.tb14347.x. [DOI] [PubMed] [Google Scholar]
- 26.Thomas C L, Gregory R J, Winslow G, Muto A, Zimmermann R A. Nucleic Acids Res. 1988;16:8129–8146. doi: 10.1093/nar/16.16.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murgola E J, Pagel F T, Hijazi K A, Arkov A L, Xu W, Zhao S Q. Biochem Cell Biol. 1995;73:925–931. doi: 10.1139/o95-100. [DOI] [PubMed] [Google Scholar]
- 28.Allen P N, Noller H F. Cell. 1991;66:141–148. doi: 10.1016/0092-8674(91)90146-p. [DOI] [PubMed] [Google Scholar]
- 29.Goldman E, Rosenberg A H, Zubay G, Studier F W. J Mol Biol. 1995;245:467–473. doi: 10.1006/jmbi.1994.0038. [DOI] [PubMed] [Google Scholar]
- 30.Irwin B, Heck J D, Hatfield G W. J Biol Chem. 1995;270:22801–22806. doi: 10.1074/jbc.270.39.22801. [DOI] [PubMed] [Google Scholar]
- 31.Shine J, Dalgarno L. Nature (London) 1975;254:34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- 32.Steitz J A, Jakes K. Proc Natl Acad Sci USA. 1975;72:4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui A, de Boer H A. Proc Natl Acad Sci USA. 1987;84:4762–4766. doi: 10.1073/pnas.84.14.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacob W F, Santer M, Dahlberg A E. Proc Natl Acad Sci USA. 1987;84:4757–4761. doi: 10.1073/pnas.84.14.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K, Holland-Staley C A, Cunningham P R. RNA. 1996;2:1270–1285. [PMC free article] [PubMed] [Google Scholar]
- 36.Sørensen M A, Pedersen S. J Mol Biol. 1991;222:265–280. doi: 10.1016/0022-2836(91)90211-n. [DOI] [PubMed] [Google Scholar]
- 37.de Smit M H, van Duin J. J Mol Biol. 1994;244:144–150. doi: 10.1006/jmbi.1994.1714. [DOI] [PubMed] [Google Scholar]
- 38.Jacques N, Dreyfus M. Mol Microbiol. 1990;4:1063–1067. doi: 10.1111/j.1365-2958.1990.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 39.Yarchuk O, Jacques N, Guillerez J, Dreyfus M. J Mol Biol. 1992;226:581–596. doi: 10.1016/0022-2836(92)90617-s. [DOI] [PubMed] [Google Scholar]
- 40.Olins P O, Rangwala S H. J Biol Chem. 1989;264:16973–16976. [PubMed] [Google Scholar]
- 41.Thanaraj T A, Pandit M W. Nucleic Acids Res. 1989;17:2973–2985. doi: 10.1093/nar/17.8.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallie D R, Kado C I. Proc Natl Acad Sci USA. 1989;86:129–132. doi: 10.1073/pnas.86.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzareva N V, Makhno V I, Boni I V. FEBS Lett. 1994;337:189–194. doi: 10.1016/0014-5793(94)80271-8. [DOI] [PubMed] [Google Scholar]
- 44.Ringquist S, Jones T, Snyder E E, Gibson T, Boni I, Gold L. Biochemistry. 1995;34:3640–3648. doi: 10.1021/bi00011a019. [DOI] [PubMed] [Google Scholar]
- 45.Samaha R R, Green R, Noller H F. Nature (London) 1995;377:309–314. doi: 10.1038/377309a0. [DOI] [PubMed] [Google Scholar]