Abstract
Aim: To determine the magnitude and causes of low vision and blindness in the Gurage zone, central Ethiopia.
Methods: A cross sectional study using a multistage cluster sampling technique was used to identify the study subjects. Visual acuity was recorded for all adults 40 years and older. Subjects who had a visual acuity of <6/18 were examined by an ophthalmologist to determine the cause of low vision or blindness.
Results: From the enumerated population, 2693 (90.8%) were examined. The prevalence of blindness (<3/60 better eye presenting vision) was 7.9% (95% CI 6.9 to 8.9) and of low vision (6/24–3/60 better eye presenting vision) was 12.1% (95% CI 10.9 to 13.3). Monocular blindness was recorded in 16.3% of the population. Blindness and low vision increased with age. The odds of low vision and blindness in women were 1.8 times that of the men. The leading causes of blindness were cataract (46.1%), trachoma (22.9%), and glaucoma (7.6%). While the prevalence of vision reducing cataract increased with age, the prevalence of trachoma related vision loss did not increase with age, suggesting that trichiasis related vision loss in this population might not be cumulative.
Conclusion: The magnitude of low vision and blindness is high in this zone and requires urgent intervention, particularly for women. Further investigation of the pattern of vision loss, particularly as a result of trachomatous trichiasis, is warranted.
Keywords: visual acuity, blindness, low vision, trachoma, cataract, refractive errors, glaucoma
The prevalence of blindness in sub-Saharan Africa varies widely. Blindness ranges from 0.3–1.3% and low vision ranges from 1.4–3.6%.1,2 The lowest blindness prevalence (<3/60 in the better eye) has been reported from Congo (0.3%),3 while, in most other settings the prevalence of blindness has been over 1.0%.1 Low vision (defined as 6/24 to 3/60) has ranged from 1.4% (Gambia) to 4% (Cameroon).4
Ethiopia (population 65 million) is one of the least developed countries in Africa with an annual per capita gross national product of US$100. The country has 58 ophthalmologists, nearly 80% of them working in the capital. There are 73 ophthalmic nurses/ophthalmic medical assistants and 11 optometry assistants. Only four to five ophthalmic medical assistants are trained in cataract surgery. In the year 2000, 22 500 cataract surgeries were performed in Ethiopia giving a cataract surgical rate (CSR) of 357/million population.
There has been no national blindness survey in Ethiopia. The available data on the prevalence of low vision and blindness in Ethiopia are mostly hospital based or studies done in focal areas of the country. In his report to the World Health Organization on an investigation carried out in 1981, Budden estimated a blindness rate of 1.5%.5 He estimated that approximately 80% of this blindness was due to preventable or curable causes. In the same year, a blindness survey in seven regions of Ethiopia for those aged 6 years and above reported a 5.1% and 1.3% prevalence rate of low vision and blindness, respectively.6 Wondu and colleagues found a blindness rate of 1.1% in central Ethiopia7 and Zerihun and Mabey8 found a blindness prevalence of 0.85% in Jimma zone. The leading causes of blindness that have been reported in Ethiopia are trachoma, cataract, glaucoma, malnutrition, and infections (the latter two mainly in children).9
We sought to conduct a population based assessment of blindness and visual impairment in Gurage zone, an area known for trachoma endemicity, to assess the relative contribution of trachoma and cataract to blindness and visual impairment. Information was collected to help plan for a regional blindness programme.
SUBJECTS AND METHODS
Study site
The study was undertaken in Gurage zone (population 1.5 million) in central Ethiopia. Gurage has three agroecological zones: dega (altitude 2500–4000 metres above sea level) estimated to account for 28.1% of the land area and 20–25% of the population, woina dega (altitude 1800–2400 metres above sea level), which accounts for 64.9% of the land area and 65–70% of the population, and kolla (altitude below 1800 metres above sea level), which accounts for 7% of the land area and 3–5% of the population. The Gurage mountains extend from the Awash river basin in the north to the Hadia zone in the south, partitioning the zone in half. The mountains form a watershed between the Gibe river basin in the west and the great east African rift valley in the east of the zone. In the zone there is one ophthalmologist and two ophthalmic medical assistants (OMAs), one of whom is also a cataract surgeon, all supported by non-governmental organisations (NGOs). According to the 1998 annual report of the two NGOs working in the zone, 455 cataract and 1753 trichiasis operations were done (GBL and Attat hospitals, 1998 report) giving a cataract surgical rate of 303 per million population.
Study population
The study was undertaken among adults (age 40 and above) as well as among children (age 1–6 years); the childhood data are not presented here. Unlike previous surveys of blindness in adults (generally including those age 50+ years) we chose to include the age group 40–49 because trachomatous low vision and blindness can be common in the age group 40–49 years.
Sample size and sampling
Multistage cluster sampling was employed to identify the study subjects. For the purpose of determining the sample size, we estimated the prevalence of blindness (<3/60) for adults 40 years and above to be 10%. We assumed a design effect of 1.5 (for cluster sampling), a confidence interval of 95%, and a response rate of 90%. These assumptions led to a sample size of 2560. Considering an average household size of 4.5 individuals, 3800 households were needed to generate the sampled adult population.
The zone has 11 districts and each district has 20–50 peasant associations (PAs). PAs in all districts were listed with their population (using the 1994 census) and 25 PAs were selected using a probability proportional to size (PPS) method. Each district had a minimum of one and a maximum of three PAs in the sample. The PA was again divided into villages having 150–200 households. All the villages in the selected PA were listed and a lottery drawn to select one village. All adults 40 years and above living in the village (for at least the past 2 months) were considered eligible for inclusion in the study. Before the start of the study village leaders were informed of the study and asked to assist with information and consent for examination. The study was approved by the regional health bureau.
All study staff were trained before initiation of the survey and a pilot test was undertaken in one village (not included in the final data) to test the procedures and the examiners. The survey coordinator (MM) supervised all aspects of the survey. There were two teams, each being led by an ophthalmologist and assisted by an ophthalmic nurse and enumerators. The steps involved in the survey were as follows. All households in the selected area were enumerated. Enumeration included name, age, and sex of each household member. Following enumeration, residents were invited to come to a central location where visual acuity was taken. Visual acuity (VA) was measured using an illiterate “E” chart in outdoor light at 6 metres. Individuals with a VA of less than 6/18 were rechecked with pinhole, and if improved, both were recorded. An ophthalmologist examined all residents with a VA of <6/18 (either eye) using an ophthalmoscope, and Schiotz tonometer to diagnose the anatomical/aetiological and principal cause of blindness and low vision. For the purpose of the survey we defined blindness as a visual acuity of less than 3/60, severe low vision as 5/60–3/60, and low vision as 6/24 to 6/60. In all cases we defined blindness or low vision as presenting vision in the better eye. Refractive errors were determined by ophthalmoscopic lens power readings, fundus findings, or observed improvement in acuity with pinhole examination. Glaucoma was considered the primary cause of vision loss or blindness in those with pathological optic disc cupping (>0.6) with increased vertical diameter in the presence of increased intraocular pressure (IOP > 21 mm Hg). Patients with corneal opacity were excluded from glaucoma assessment. As one or more principal causes of blindness/low vision could be diagnosed per person and per eye, we chose the treatable cause as the principal cause.
All cases with VA of <6/18 were also interviewed on past history of cataract and trichiasis surgery. Cases with treatable conditions were referred to the nearest health unit and, at a later date, integrated eye care workers conducted trichiasis surgery. A mobile team led by an ophthalmologist was organised for the cataract surgeries and other treatable conditions.
Data analysis
All data was included on epi-info version 6 and univariate analysis was undertaken to assess prevalence and causes of blindness by sex. χ2 and odds ratios were calculated. For analysis of the pattern of vision loss among men and women we used eyes as the unit of analysis.
RESULTS
Overall, 2964 individuals aged ≥40 years were enumerated. There were more men than women in the oldest age groups (2.3:1 in the 70+ age group and 1.1:1 in the 60–69 age group). Vision status was determined in 2693 (90.8%), 234 (8.9%) of whom were aided with spectacle correction. Among the 271(9.2%) individuals without vision status assessment males between 40–49 predominated (data not shown). Visual acuity was measured using Snellen charts for 98% of examined patients while visual status was grossly assessed for the rest by asking them to count outstretched fingers at a distance of 3 metres. The gross assessment was necessary for those bed ridden or too old to come to the examination site, and who were therefore assessed in their own households. Among those who had gross assessment of vision 50 (92.6%) were believed not blind and four (7.4%) were blind.
Overall, 2113 (80.1%) individuals aged 40 years and above had good vision (a visual acuity (VA) of ≥6/18 in the better eye) with available correction. Of these individuals, 1790 (84.7%) had a binocular VA of ≥6/18; the remaining 323 (15.3%) had monocular blindness or visual impairment and a VA of ≥6/18 in the other eye with the available correction. Bilateral blindness was recognised in 208 (7.9%) individuals, severe visual impairment (5/60–3/60) in 60 individuals (2.3%), and low vision (6/24–6/60) in 258 individuals (9.8%). Overall, 442 residents (16.3%) had monocular blindness. As expected, blindness and low vision increased with age (Table 1).
Table 1.
Visual acuity by age and sex in Gurage zone
| Age group (years) | |||||
| Visual acuity in the better eye adjusted | 40–49 No (%) | 50–59 No (%) | 60–69 No (%) | 70+ No (%) | Age |
| Men: | |||||
| ≥6/18 | 425 (90.8) | 305 (85.2) | 208 (78.9) | 80 (58.3) | 83.5% |
| 6/24–6/60 | 23 (4.9) | 25 (7.0) | 26 (9.8) | 16 (11.7) | 7.1% |
| 5/60–3/60 | 4 (0.9) | 4 (1.1) | 7 (2.7) | 7 (5.1) | 1.7% |
| <3/60 | 16 (3.4) | 24 (6.7) | 23 (8.7) | 34 (24.8) | 7.7% |
| Total | 468 | 358 | 264 | 137 | |
| Women: | |||||
| ≥6/18 | 584 (86.1) | 334 (76.1) | 151 (64.3) | 26 (43.3) | 75.5% |
| 6/24–6/60 | 59 (8.7) | 65 (14.8) | 33 (14.0) | 11 (18.3) | 12.2% |
| 5/60–3/60 | 12 (1.8) | 11 (2.5) | 13 (5.5) | 2 (3.3) | 2.8% |
| <3/60 | 23 (3.4) | 29 (6.6) | 38 (16.2) | 21 (35.0) | 9.5% |
| Total | 678 (100) | 439 (100) | 235 (100) | 60 (100) | 100% |
While the crude rate of blindness was the same for men and women (7.9%) this was primarily due to the fact that the age specific rate of blindness for those <60 (74% of our sample) was similar for men and women. In the older age groups blindness or visual impairment was 1.5 to 2 times higher in females compared to males (Table 2). Controlling for age, the odds of low vision and blindness was 1.8 times higher (95% CI 1.5 to 2.2) in females than males (Mantel-Haenszel χ2 =31.2; p<0.001). Among the younger age groups (40–49 and 50–59) there was excess visual impairment (<6/24–6/60) and severe visual impairment (<6/60) in women.
Table 2.
Excess risk of visual impairment and blindness in women compared to men (odds ratio and 95% confidence interval)
| Odds ratio and 95% CI of excess vision loss in women compared to men | |||
| Presenting better eye vision | |||
| Age group | <3/60 | <6/60 | <6/18 |
| 40–49 | 0.99 (0.50 to 1.99) | 1.22 (0.67 to 2.22) | 1.59 (1.07 to 2.37) |
| 50–59 | 0.98 (0.54 to 1.66) | 1.18 (0.69 to 2.02) | 1.81 (1.24 to 2.65) |
| 60–69 | 2.02 (1.13 to 3.64) | 2.16 (1.29 to 3.64) | 2.07 (1.36 to 3.14) |
| 70+ | 1.63 (0.80 to 3.3) | 1.46 (0.73 to 2.88) | 1.96 (1.02 to 3.81) |
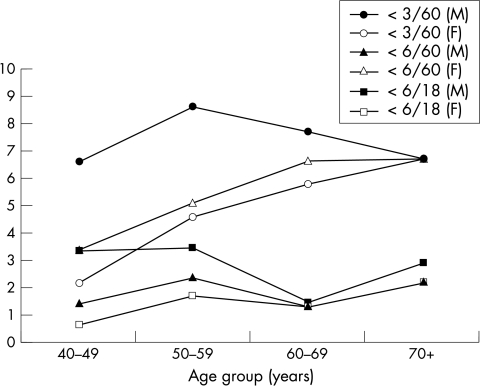
The leading principal causes of visual impairment, severe visual impairment, and blindness were cataract, trachoma, refractive errors, glaucoma, and uncorrected aphakia (Table 3). In the younger age group (40–49 years) trachoma was the leading cause of visual impairment, severe visual impairment, and blindness (26.4% in males and 38.3% in females). In men cataract was the second leading cause (26.4%) of visual impairment while in women refractive errors (29.2%) was the second leading cause. In the age group 50–59, cataract was the leading cause of visual impairment and blindness in both sexes (51.5% for men and 44.6% for women). Trachoma was the second leading cause (women 31% and men 18.4%) followed by refractive errors (10.3% in men and 9.1% in women). In ages 60–69 the proportion of cataract increased (61.2% in men and 55.0% in women) and trachoma decreased to 5.3% in men and 18.0% in women. In the oldest age group, 70+, 52.8% in men and 69.1% in women had cataract leading to blindness or visual impairment and only 4.9% of men and 11.8% of women had visual loss attributed to trachoma. In all age groups, women accounted for 58.2% of the people with visual impairment, severe visual impairment, and blindness. As expected, the prevalence of cataract induced vision loss increased with age, remaining higher among women than men (Figs 1 and 2) Assuming that uncorrected aphakia as a cause of visual impairment represents cataract surgical coverage (IOL implantation is not available in the zone and aphakic spectacles are generally unavailable), the cataract surgical coverage can be estimated as 7.8%, 10.5% among men and 5.2% among women. The prevalence of trachoma induced vision loss did not increase with age; women had a higher prevalence than men in all age groups (Fig 3).
Table 3.
Causes of low vision and blindness
| Visual acuity | |||
| Cause | 6/24–6/60 | 5/60–3/60 | <3/60 |
| Cataract | 176 (33.7%) | 62 (46.3%) | 354 (50.4%) |
| Trachoma | 109 (20.9%) | 39 (29.1%) | 137 (19.5%) |
| Refractive errors | 133 (25.5%) | 14 (10.4%) | 25 (3.5%) |
| Glaucoma | 16 (3.1%) | 1 (0.7%) | 54 (7.7%) |
| Uncorrected aphakia | 3 (0.6%) | 4 (3.0%) | 23 (3.3%) |
| Others | 85 (16.3%) | 14 (10.4%) | 109 (15.5%) |
Figure 1.
Age specific prevalence of visual impairment due to cataract and trachoma (by eye).
Figure 2.
Cataract as a cause of vision loss and blindness (by eye).
Figure 3.
Trachoma as a cause of vision loss and blindness (by eye).
DISCUSSION
The prevalence of blindness (7.9%) and visual impairment (12.1%) in those 40 years and above in Gurage was high. Blindness was 1.5 to 5.1 times higher in Gurage zone compared to the recent Jimma8 zone study (Table 4). Consistent with the findings from a meta-analysis of blindness surveys, women had a higher prevalence of blindness compared to men.10 Unlike other settings in developing countries, men outnumbered women in the oldest age group by 2 to 1. This unusual age-sex pattern was confirmed by follow up but could not be explained adequately.
Table 4.
Comparison of age specific blindness (<3/60 better eye presenting) prevalence rates (per 1000 population) in Jimma8 and Gurage, Ethiopia
| Age group | Jimma | Gurage | Excess blindness in Gurage |
| 40–49 | 7.16 | 34.0 | 4.7 |
| 50–59 | 13.1 | 66.5 | 5.1 |
| 60–69 | 83.0 | 122.2 | 1.5 |
| 70+ | 175.2 | 279.2 | 1.6 |
The three leading causes of bilateral blindness identified in our study were cataract, trachoma, and glaucoma. The three leading causes of low vision were cataract, trachoma, and refractive errors, similar to findings reported elsewhere in Ethiopia.6 Interestingly, while cataract increased with age, trachoma related blindness did not. This pattern could be explained in several ways. Firstly, it is possible that trichiasis is being operated upon before vision loss has occurred. Routine high volume trichiasis surgery has been only recently introduced in Gurage and, in 2001, 5120 trichiasis surgeries were done in the zone. It has been estimated that there are 45 000 people with trichiasis in Gurage, giving a trichiasis surgical coverage of 12%. A large proportion of trichiasis surgical cases are in people who have come from outside Gurage zone and that a large proportion are in individuals who already have significant vision loss (Attat Hospital, personal communication). Therefore, it is unlikely that this explains the pattern. A second explanation would be that trachoma related vision loss and blindness is on the increase and we are observing the variable impact of trachoma on different age cohorts. However, trachoma is hyperendemic in Gurage and there is no evidence of an increase in active disease or its sequelae. A third explanation would be that visual impairment and blindness secondary to trichiasis are not inevitable, but are dependent on events that predispose to rapid ulceration and scarring. The anatomical position of the inturned eyelashes is likely to be more critical to causing corneal damage than the number of lashes.11 Furthermore, recent research from the Gambia has shown that the incidence of blindness due to trachoma is relatively rare; in the Gambia only 2.5% of individuals with trichiasis developed visual impairment or blindness in a 12 year period.12 Further investigation of this pattern of vision loss and blindness due to trachoma is warranted.
As in most developing countries the 70+ age group, while the smallest, is increasing the most rapidly. The high rate of blindness in the oldest age groups in Gurage zone (25% of men and 35% of women 70+ years of age with vision <3/60) suggests that efforts are needed to promote blindness prevention and treatment as a programme for the most elderly. The higher rates of visual impairment and blindness in women compared to men are due to the higher rates of both unoperated cataract and trachomatous trichiasis in women than men. Specific efforts are needed to direct eye care service promotion and delivery to women in order to significantly reduce blindness and visual impairment in these communities.
Acknowledgments
The research was supported by Silvia Adams Trust to whom we are grateful. We thank the SNNPR Health Bureau and the Gurage Health Department for facilitating this study and we are grateful to all the Peasant Association leaders for giving their time and assistance for the study. Lastly, the study was made possible by the full support of ORBIS International/New York.
REFERENCES
- 1.Lewallen S, Courtright P. Blindness in Africa: present situation and future needs. Br J Ophthalmol 2001;85:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thylefors B, Negrel A-D, Pararajasegaram R, et al. Global data on blindness. Bull World Health Organ 1995;73:115–21. [PMC free article] [PubMed] [Google Scholar]
- 3.Thylefors B, Negrel A-D, Pararajasegaram R, et al. Avoidable blindness. WHO/PBL/94.38. Geneva: WHO update, 1994.
- 4.Thylefores B, Negrel A-D, Pararajasegaram R, et al. Available data on blindness. Ophthalmic Epidemiol 1995;2:5–39. [DOI] [PubMed] [Google Scholar]
- 5.Budden FH. A report on blindness in Ethiopia. Geneva: WHO, STC, 1981.
- 6.Cerulli L, Cedrone C, Cherinet A, et al. Assessment of the visual status of the population of seven regions in Ethiopia. Rev Int Trach 1984:127–35. [PubMed]
- 7.Wondu A, Redda T, Forsgren L, et al. Causes of visual impairment in central Ethiopia. Ethiopian Med J 1995;33:163–74. [PubMed] [Google Scholar]
- 8.Zerihun N, Mabey D. Blindness and low vision in Jimma Zone, Ethiopia: results of a population-based survey. Ophthalmic Epidemiol 1997;4:19–26. [DOI] [PubMed] [Google Scholar]
- 9.Wondu A, Cherinet A. Eye diseases and blindness in Ethiopia. In: Kloos, Zein, eds. Health and disease in Ethiopia. West View Press, 1993.
- 10.Abou-Gareeb I, Lewallen S, Bassett K, et al. Gender and blindness: a meta-analysis of population based prevalence surveys. Ophthalmic Epidemiol 2001;8:39–56. [DOI] [PubMed] [Google Scholar]
- 11.Melese M, Alemayehu W, Bejiga A, et al. Modified grading system of upper eyelid trachomatous trichiasis. Ophthalmic Epidemiol (in press) [DOI] [PubMed]
- 12.Bowman RJ, Faal H, Myatt M, et al. Longitudinal study of trachomatous trichiasis in the Gambia. Br J Ophthalmol 2002;86:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]