Abstract
Background: In patients with Graves’ disease, smoking considerably increases the incidence and severity of thyroid associated ophthalmopathy (TAO). The authors sought to determine if smoking also influences the course of TAO during treatment, and the efficacy of therapy.
Methods: 41 smokers and 19 non-smokers with moderate untreated TAO were included in this prospective study. All patients were treated with steroids and, 6 weeks after the beginning of drug therapy, with orbital irradiation. Follow up was performed 1.5, 4.5, 7.5, and 12 months after the beginning of the study. Proptosis, clinical activity score (CAS), and motility were evaluated. The extent of smoking was derived from the concentration of the haemoglobin adduct N-2-hydroxyethylvaline (HEV), a parameter of long term smoking.
Results: There was no difference in the clinical manifestations of TAO between smokers and non-smokers at the beginning of treatment. However, CAS decreased (p<0.05) and motility improved (p<0.02) significantly faster and to a greater extent in non-smokers than smokers. Inverse correlations between the CAS decrease and the HEV levels observed 4.5 and 7.5 months after the beginning of treatment and between the improvement of motility and the HEV levels after 1.5, 4.5, and 7.5 months indicated a dose dependence. Mean HEV levels did not vary much during the follow up period and were significantly different in smokers (mean 5.4 (SD 2.7) μg/l) and non-smokers (mean 1.8 (1.3) μg/l; p<0.01).
Conclusion: Smoking influences the course of TAO during treatment in a dose dependent manner. The response to treatment is delayed and considerably poorer in smokers.
Keywords: thyroid associated ophthalmopathy, smoking, haemoglobin adducts
Thyroid associated ophthalmopathy (TAO) occurs more frequently and tends to be more severe in smokers than non-smokers.1–8 Current but not lifetime tobacco use correlates with the severity of TAO.8 A prospective study revealed that smoking increases the risk of ophthalmopathy progression after radioiodine therapy.9 A retrospective study showed that smoking decreases the efficacy of orbital irradiation and of glucocorticoid therapy.9 According to another retrospective investigation, however, the final outcome does not seem to be influenced by smoking habits.10
In this prospective study, we investigated the influence of smoking on the response to treatment during the course of TAO. Tobacco use was evaluated by quantitative analysis of the haemoglobin adduct N-2-hydroxyethylvaline (HEV). This approach allows us to evaluate the dose dependence of the therapeutic response from tobacco consumption. HEV is formed from hydroxyethylating agents of cigarette smoke and reflects the integrated “smoking dose” over the lifetime of erythrocytes. Although other sources of ethylene oxide (that is, exhaust fumes, endogenous ethylene production11) influence the HEV concentration, a linear relation between the number of cigarettes smoked per day and the HEV levels has been observed (correlation coefficient r = 0.54) indicating that HEV is a suitable biomarker for active and passive smoking.12
PATIENTS AND METHODS
Patients
The prospective study included 67 patients with active TAO of moderate severity (manifestation less than 12 months before the beginning of the study). Sixty patients completed a 1 year follow up period. The data of these patients were statistically evaluated. Five patients were lost during the follow up period owing to a lack of compliance or a change of residence. Two were excluded because of radioiodine therapy. Among the patients who completed the study, 41 were smokers (11 men and 30 women, mean age 46 (18–70) years) and 19 non-smokers (four men and 15 women, mean age 47 (30–69) years).
Thyroid status of the patients was mainly euthyroid with thyrosis stimulating hormone (TSH) still being suppressed under antithyroid therapy except for a few patients presenting with overt hyperthyroidism or hypothyroidism (because of overtreatment). Thyroidal function was not significantly different in smokers and non-smokers at the time of the initial presentation (Table 1) and during the course of the study (data not shown). TSH receptor antibodies were elevated in the majority of patients (n=58) with no significant difference in titre between the groups (Table 1). Two smokers and one non-smoker underwent thyroidectomy, two patients received radioiodine treatment.
Table 1.
Baseline characteristics of the smokers (HEV >2.9 μg/l) and non-smokers (HEV <2,9 μg/l) obtained in the initial examination
| Feature | Non-smokers (n=19) | Smokers (n=41) | p Value |
| GD since (months) | 14 (0–60) | 16 (3–75) | NS |
| TAO since (months) | 5 (0–12) | 6 (3–12) | NS |
| CAS score | 3.6 (2–6) | 3.8 (2–6) | NS |
| Motility score | 0.9 (0–3) | 1.2 (0–3) | NS |
| Proptosis (mm) | 17.4 (11–22) | 17.6 (11–24) | NS |
| HEV level (μg/l) | 1.5 (0.4–2.7) | 5.6 (2.8–13.9) | p<0.001 |
| TSH (mU/l) | 0.1 (<0.1–19.8) | 0.08 (<0.1–6.7) | NS |
| free T4 (pmol/l) | 16.5 (10.2–23.2) | 16.0 (8.8–27.5) | NS |
| T3 (nmol/l) | 2.0 (1.4–2.6) | 2.4 (1.5–5.9) | NS |
| T4 (nmol/l) | 103.5 (73.2–163.2) | 112.0 (61.4–190.7) | NS |
| TSHRAb (U/l) | 14.0 (<5–145) | 14.0 (<5.0–141) | NS |
GD = Graves’ hyperthyoidism, TAO = thyroid associated ophthalmopathy, CAS = clinical activity score, HEV = N-2-hydroxyethylvaline, TSH = thyroid stimulating hormone, T4 = l-thyroxine, T3 = tri-iodothyronine, THS-R-Ab = TSH receptor antibodies). Median values, 5% and 95% confidence intervals are presented.
All patients were treated with steroids (fluorocortolone 100 mg reduced by 10 mg every 4 days) for 6 weeks and, after that, with orbital irradiation (12 Gy). They were examined at the beginning of the study and after 6 weeks, 4.5, 7.5, and 12 months, respectively. Patients with mild TAO (clinical activity score <2), severe TAO (proptosis >24 mm, reduced visual acuity due to optic nerve compression), a TAO duration of more than 12 months, former anti-inflammatory treatment, and radioiodine therapy during the follow up period were excluded from the study.
All follow ups were carried out by the same investigator who assessed the following ophthalmological parameters:
Proptosis (mm) was measured, using the Hertel method.
The CAS was assessed as follows (we modified the scheme of Mourits13): pain during eye movements (0–1), painful oppressive feeling on or behind the eye (0–1); lower (0–2) and upper eyelid oedema (0–2); conjunctival injection (0–1); conjunctival chemosis (0–1). This resulted in a maximal score of 8.
Eye motility (score 0–3) was evaluated by measuring monocular excursion at the Goldmann perimeter: no impairment (0), impaired monocular elevation to 25°–35° and/or impaired monocular abduction to 35°–40° (1), impaired monocular elevation to 15°–24° and/or impaired monocular abduction to 30°–34° (2), impaired monocular elevation below 15° and/or impaired monocular abduction below 30° (3).
Improvement upon treatment was presumed, if the CAS was less than 2 (mean of both eyes), and/or proptosis was reduced by more than 2 mm (mean of both eyes), and/or motility improved from score 2 or 3 to score 0 or 1.
The characteristics of the patients at the beginning of the study are given in Table 1. All patients had a CAS >2 at the first visit. According to the patients, the first TAO symptoms (increase of lid width, a proptosis of eye balls, and oedema) appeared not earlier than 12 months before the initial examination. 27 patients had an impaired motility judged as grade 2 or 3.
Biochemical measurements
Biochemical parameters were determined by an immunometric assay (ACS, 180 Cheiron, Fernwald, Germany) with reference ranges for TSH of 0.3–4 mIU/l, for serum FT4 of 10–25 pmol/l, for T4 of 58–154 nmol/l, and for T3 of 1.23–3.08 nmol/l. Patients receiving antithyroid drug treatment were regularly tested for euthyroidism. Patients with elevated TSH and/or FT3 were either treated with an adjusted dosage of levothyroxine or the doses of antithyroid drugs were reduced.
TSH receptor antibodies (TSHRAb) were measured by the first generation TSH receptor assay (Brahms, Berlin, Germany).
Determination of N-2-hydroxyethylvaline (HEV) as evidence of exposure to ethylene oxide
Each visit included the quantitative analysis of the haemoglobin adduct N-2-hydroxyethylvaline (HEV). HEV was quantified as 1-(2-hydroxyethyl)-5-isopropyl-3-pentafluorophenyl-2-thiohydantoin derivative by gas chromatography/mass spectrometry (GC/MS) following isolation of the globin from haemolysed erythrocytes, derivatisation with pentafluorophenyl isothiocyanate, and cleavage from the protein by means of a modified Edman degradation procedure.14
A patient was considered a smoker if HEV levels were above 2.9 μg/l, and a non-smoker if HEV levels were below 2.9 μg/l. The mean HEV value in non-smokers (all measurements) was 1.8 (+1.3) = 3.1 μg/l, the mean HEV value in smokers was 5.4 (−2.7) = 2.7 μg/l; 2.9 is the mean of these two values.
Statistical analysis
Differences between features of patients with HEV levels above 2.9 μg/l (smokers) and those of patients with levels below 2.9 μg/l (non-smokers) at the beginning of treatment were calculated with the unpaired two tailed t test. The response to treatment is presented via life table analysis according to Kaplan-Meier. The differences between smokers and non-smokers were tested with the log rank test 12 months after the beginning of the study. We calculated Spearman’s correlation coefficients for the relation between the improvement of symptoms and the HEV levels measured at the time of the consecutive examinations. The difference between the score at the beginning of the treatment and the score assessed in each follow up examination indicated the improvement of symptoms. A p<0.05 was considered significant.
Medical ethics committee
The study was approved by the medical ethics committee of the University of Essen, Germany.
RESULTS
Clinical assessment before treatment
Owing to the exclusion of patients with mild and severe TAO, there was no significant difference between the clinical manifestations in smokers and non-smokers observed in the first examination, also, thyroid hormone status and antibody levels were similar in the two groups (Table 1).
HEV levels and smoking habits
The HEV levels of smokers were significantly different from those of non-smokers in all examinations. However, the respective mean values in both groups did not vary much during the follow up period, indicating no change in smoking habits. The number of cigarettes smoked per day correlated positively with the HEV levels throughout the follow up period (r = 0.56–0.60; p<0.001). Three non-smokers with HEV levels above 2.9 μg/l insisted on having quit smoking, but claimed to have continued substantial passive exposure to tobacco smoke.
Improvement of TAO symptoms in the course of treatment
Soft tissue injury (as judged by the CAS) responded best to the anti-inflammatory treatment, both in smokers and in non-smokers. In contrast, eye motility improved less favourably, and proptosis did not change significantly in both groups.
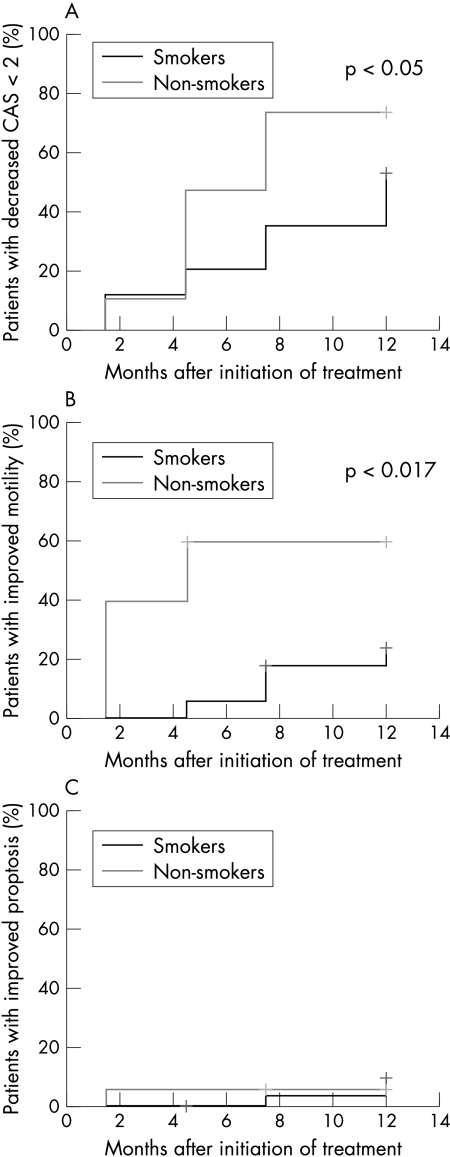
Kaplan-Meier curves (Fig 1) revealed, however, that smokers responded to a minor extent and more protractedly to the treatment than non-smokers.
Figure 1.
Kaplan-Meier-analysis indicating the improvement of the clinical symptoms during the observation period of 12 months. (A) Improvement of clinical activity as assessed by the reduction to score 1 or 0. Clinical activity has decreased in 74% of the non-smokers and in 53% of the smokers to score 1 or 0 after 12 months (p<0.05). (B) Improvement of motility as assessed by a reduction from score 3 or 2 to score 1–0. Motility has improved in 60% of the non-smokers and in 24% of the smokers after 12 months (p<0.017). (C) Improvement of proptosis defined as reduction of more than 2 mm. Proptosis has decreased more than 2 mm in 5% of the non-smokers and in 9% of the smokers after 12 months. The difference was not significant.
The CAS (Fig 1A) has decreased to 1 or 0 in almost half of the non-smokers 4.5 months after the beginning of the study; the corresponding quota in smokers was 21%. After 12 months, the CAS has decreased to 1 or 0 in 74% of the non-smokers, but only in 55% of the smokers. The log rank test revealed a statistical difference between these two groups for the respective scores after 12 months (p<0.05).
The improvement of monocular elevation to 25° or more and/or monocular abduction to 35° or more in smokers and non-smokers is depicted in Figure 1B. While five non-smokers and 22 smokers showed an impaired motility score of 2 or 3 at the beginning of the study, the score of three non-smokers and four smokers has improved to score 0 or 1 a year later. The difference between the two groups was statistically significant (p<0.017).
Reduction of proptosis indicating improvement was rather poor in both groups. Three smokers and one non-smoker showed a reduction by more than 2 mm after 12 months. The difference between the two groups was not significant (Fig 1C).
Improvement of TAO symptoms upon treatment dependence on the extent of smoking
Table 2 shows the respective Spearman’s correlation coefficients between the improvement of clinical symptoms as indicated by the CAS and the corresponding HEV levels. The reduction of clinical activity after 4.5 and 7.5 months and the improvement of motility after 1.5, 4.5, and 7.5 months correlated significantly with the corresponding HEV levels. No significant dependence on the extent of smoking was found for the outcome after 1 year.
Table 2.
Relation between improvement (CAS, motility, and proptosis) and HEV levels
| Months | CAS | Motility | Proptosis |
| 0 | −0.03 | −0.04 | −0.03 |
| 1.5 | −0.16 | −0.34 | −0.03 |
| 4.5 | −0.29 | −0.33 | −0.12 |
| 7.5 | −0.30 | −0.29 | −0.08 |
| 12 | −0.12 | −0.23 | −0.08 |
Significant correlations are indicated in bold.
DISCUSSION
This prospective study revealed a dependence of the therapeutic response to anti-inflammatory therapy on the extent of smoking in TAO patients only in the first months (1.5–7.5) after the beginning of treatment. No significant dose dependence was found for the outcome after 1 year, however, although Kaplan-Meier analysis still showed a significantly smaller reduction of clinical activity and a poorer improvement of motility in smokers than non-smokers at that time.
Relation between HEV levels and the number of smoked cigarettes
The use of cigarettes was reliably estimated by the quantification of HEV in haemoglobin. There was a significant correlation between the average number of cigarettes smoked per day according to the patients and the HEV levels. The correlation coefficients from r = 0.56 to 0.60 are almost identical to those obtained by Bailey,12 and only slightly below the respective coefficient of r = 0.63 calculated by Bono et al.15 Bono et al also considered the contribution of passive smoking, which might explain the higher value obtained in their investigation. Thus, the method employed in this study proved to be suitable to estimate the influence of smoking on the response to TAO therapy and to investigate the dependence of this response on the extent of smoking.
Dependence of the improvement of TAO symptoms during treatment on the extent of smoking
Soft tissue inflammation as expressed by the CAS responded best to the anti-inflammatory therapy: approximately two thirds of the patients showed a reduced CAS 1 year after the beginning of the study. The improvement of motility was only moderate (observed in one third of the patients) and a slight reduction of proptosis occurred only in a few patients. The significant correlation between the reduction of clinical activity and motility and the HEV levels 1.5–7.5 months after the beginning of drug therapy indicates a direct effect of smoking habits on the response to treatment. The more extensive the use of cigarettes, the less favourable is the initial response to treatment. A similar dose-effect relation has also been found for the prevalence5 and severity of TAO.8 The fact that no such correlation was observed 12 months after the beginnung of treatment indicates that the response to treatment is only delayed in smokers. These results are in agreement with those of our previously conducted retrospective study in which the final outcome of TAO was evaluated 280–1675 days after the first examination.10 That former study had revealed that at the first visit smokers showed oedema (p<0.02) and proptosis (p<0.05) more often than non-smokers, whereas the prevalence and severity of other eye symptoms did not differ in both groups. In the course of treatment, a clear amelioration of symptoms was observed in both groups independent of smoking habits. Obviously, smoking did not adversely affect the final outcome of treatment after the longer observation period.
None of the smokers stopped smoking after having received information on its detrimental effects on TAO. Therefore, it was not possible to demonstrate that quitting smoking has indeed a beneficial effect. Such an effect can be assumed, however, if the relation between HEV levels and the improvement of TAO is taken into consideration.
The pathological influence of smoking on thyroid function, the immune system, and vascular and connetive tissue has been discussed in detail in former studies.16–21
In summary, the determination of HEV is a suitable method to estimate the intensity of smoking. Smokers with TAO respond less favourably to steroids and to radiotherapy and represent a group of patients suffering from a protracted course of the disease in the first year after the initiation of treatment. The effect of smoking on the initial response to anti-inflammatory treatment is dose dependent, but it does not influence the final outcome. Additional studies are required to elucidate the prognostic relevance of quitting smoking on the clinical course of Graves’ disease.
Acknowledgments
We thank Ms Katrin Rensing (Institute for Medical Informatics, Biometry and Epidemiology at the University of Essen) for her expert advice concerning the statistical evaluation of our data.
Presented in part at the 72nd Annual Meeting of the American Thyroid Association, Palm Beach, FL, USA 1999.
REFERENCES
- 1.Hagg E, Asplund K. Is endocrine ophthalmopathy related to smoking? BMJ 1987;295:634–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartalena L, Martino E, Marcocci C, et al. More on smoking habits and Graves’ ophthalmopathy. J Endocrinol Invest 1989;12:733–7. [DOI] [PubMed] [Google Scholar]
- 3.Balazs C, Stenszky V, Farid NR. Association between Graves’ ophthalmopathy and smoking. Lancet 1990;336:754. [DOI] [PubMed] [Google Scholar]
- 4.Shine B, Fells P, Edwards OM, et al. Association between Graves’ ophthalmopathy and smoking. Lancet 1990;335:1261–3. [DOI] [PubMed] [Google Scholar]
- 5.Tellez M, Cooper J, Edmonds C. Graves’ ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol 1992;36:291–4. [DOI] [PubMed] [Google Scholar]
- 6.Prummel MF, Wiersinga WM. Smoking and risk of Graves’ disease. JAMA 1993;269:479–482. [PubMed] [Google Scholar]
- 7.Winsa B, Mandahl A, Karlsson FA. Graves’ disease, endocrine ophthalmopathy and smoking. Acta Endocrinol (Copenh) 1993;128:156–60. [DOI] [PubMed] [Google Scholar]
- 8.Pfeilschifter J, Ziegler R. Smoking and endocrine ophthalmopathy:impact of smoking severity and current vs lifetime cigarette consumption. Clin Endocrinol 1996;45:477–81. [DOI] [PubMed] [Google Scholar]
- 9.Bartalena L, Marcocci C, Tanda ML, et al. Cigarette smoking and treatment outcomes in Graves’ ophthalmopathy. Ann Intern Med 1998;129:632–5. [DOI] [PubMed] [Google Scholar]
- 10.Quadbeck B, Kalinski R, Eckstein A, et al. Lack of influence of smoking on the course of Graves’ ophthalmopathy under antiinflammatory therapy. 71th Annual Meeting of the American Thyroid Association. Portland, Oregon, USA. The Proceedings of the ATA, 1998:S-83.
- 11.Farmer PB, Cordero R, Autrup H. Monitoring human exposure to 2-hydroxyethylating carcinogens. Environ Health Perspect 1996;104(Suppl 3):449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey E, Brooks AGF, Dollery CT, et al. Hydroxyethylvaline adduct formation in haemoglobin as a biological monitor of cigarette smoke intake. Arch Toxicol 1988;62:247–53. [DOI] [PubMed] [Google Scholar]
- 13.Mourits MP, Prummel MF, Wiersinga WM, et al. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol 1997;47:9–14. [DOI] [PubMed] [Google Scholar]
- 14.Van Sittert NJ. N-2-Cyanoethylvaline, N-2-hydroxyethylvaline, N-methylvaline (as evidence of exposure to acrylonitrile, ethylene oxide as well as methylating agents). In: Angerer J, Schaller KH, eds (on behalf of the Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area of the Deutsche Forschungsgemeinschaft). Analyses of hazardous substances in biological materials. Vol 5 VCH Verlagsgesellschaft Weinheim, 1997:181–210.
- 15.Bono R, Vincenti M, Meineri V, et al. Formation of N-(2-hydroxyethyl)valine due to exposure to ethylene oxide via tobacco smoke:A risk factor for onset of cancer. Environ Res 1999;81:62–71. [DOI] [PubMed] [Google Scholar]
- 16.Utiger RD. Effects of smoking on thyroid function. Eur J Endocrinol 1998;138:368–9. [DOI] [PubMed] [Google Scholar]
- 17.Muller B, Zulewski H, Huber P, et al. Impaired action of thyroid hormone associated with smoking in women with hypothyroidism. N Engl J Med 1995;333:964–9. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe RA, Weetman AP. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia:possible role in thyroid-associated ophthalmopathy. Clin Endocrinol 1994;40:67–72. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz J, Weiss ST. Cigarette smoking and peripheral blood leukocyte differentials. Ann Epidemiol 1994;4:236–42. [DOI] [PubMed] [Google Scholar]
- 20.McAllister-Sistilli CG, Caggiula AR, et al. The effects of nicotine on the immune system. Psychoneuroendocrinology 1998;23:175–87. [DOI] [PubMed] [Google Scholar]
- 21.Hofbauer LC, Muhlberg T, Koenig A, et al. Soluble interleukin-1 receptor antagonist serum levels in smokers and nonsmokers with Graves’ ophthalmopathy undergoing orbital radiotherapy. J Clin Endocrinol Metab 1997;82:2244–7. [DOI] [PubMed] [Google Scholar]