Abstract
Aims: To evaluate abnormalities in the choroidal circulation in cases of central serous chorioretinopathy (CSC).
Methods: A complete clinical ophthalmological examination was performed using simultaneous fluorescein and indocyanine green (ICG) angiography with a confocal scanning laser ophthalmoscopy and the digital images analysed in 36 consecutive patients with acute CSC. To quantify the choroidal circulation, the foveal choroidal blood flow was measured in 11 patients using laser Doppler flowmetry.
Results: Fluorescein angiography showed focal leakage from the retinal pigment epithelium in all patients. ICG angiography revealed delays in arterial filling in 27 eyes (75%), and fluorescein angiography showed small hypofluorescent points around the leakage in 27 eyes (75%). Abnormal choroidal hyperfluorescence was observed in 30 eyes (83%). The choroidal blood flow in eyes with CSC was 45% lower than in fellow eyes (p<0.01).
Conclusion: Decreased choroidal blood flow in CSC was demonstrated for the first time. The decreased choroidal blood flow might be correlated with the small, localised hypofluorescent areas, which may indicate non-perfused areas of the choriocapillaris that are frequently seen during ICG angiography.
Keywords: central serous chorioretinopathy, fluorescein angiography, indocyanine green angiography, foveal choroidal blood, laser Doppler flowmetry
Central serous chorioretinopathy (CSC) is characterised by a focal serous detachment of the neurosensory retina. Fluorescein angiography shows dye leakage from the retinal pigment epithelium (RPE) and subretinal dye pooling. However, fluorescein angiography has not been useful in determining the pathogenesis of CSC because of limitations in imaging the choroidal vessels. Thus, while the clinical features of CSC have been described, its pathogenesis is controversial.1–5
Numerous recent reports have described abnormalities of the choroidal circulation using indocyanine green (ICG) angiography.6–13 ICG is a dye that has several advantages over sodium fluorescein for choroidal angiography, in that it binds tightly to plasma proteins and thus prevents marked leakage from fenestrated vessels such as the choriocapillaris. The dye also absorbs and fluoresces in the near infrared range, which enhances visualisation of the fluorescence through haemoglobin, RPE, or xantophyll. Using ICG angiography, choroidal vascular abnormalities, such as filling delays of the choroidal arteries and choriocapillaris,6,10,12 venous dilatation,10,12 and focal hyperfluorescence of the choroid,6–12 which indicate hyperpermeability of choroidal vessels, have been reported.
In the present study, we performed simultaneous ICG and fluorescein angiography using scanning laser ophthalmoscopy14 in patients with CSC. We observed delayed arterial filling and hyperfluorescence of the choroid, which had been reported previously. In addition, small, localised hypofluorescent areas around the leaking point found in fluorescein angiography were frequently observed by ICG angiography. To assess the quantitatively analysis of the choroidal circulation, measurements of the choroidal blood flow in the centre of the fovea were performed in some of the patients with CSC. Interestingly, the choroidal blood flow in eyes with CSC was significantly lower than in fellow eyes. We hypothesised that the hypofluorescent area disclosed by ICG angiography might be associated with the non-perfused choriocapillaris. This choroidal circulatory disturbance may cause hyperpermeability of the choroidal vessels and/or decreased choroidal blood flow.
PATIENTS AND METHODS
We examined 36 consecutive patients (30 men, six women) with acute CSC. The patients’ ages ranged from 30–73 years (mean 45 (SD 11) years). Eyes that also had another disease such as preretinal membrane, drusen, or age related macular degeneration were excluded from the study. Informed consent was obtained from participants in accordance with the tenets of the Declaration of Helsinki. In each patient, we performed a complete clinical ophthalmological examination and simultaneous fluorescein and ICG angiography with a confocal scanning ophthalmoscope (Heidelberg Retinal Angiograph; Heidelberg Engineering, Dossenheim, Germany).14 For simultaneous fluorescein and ICG angiography, 25 mg of ICG (Daiichi Pharmaceutical Co, Ltd, Tokyo, Japan) were dissolved in 5 ml of a 20% fluorescein solution (Alcon, Tokyo, Japan) and injected into the cubital vein. For fluorescein angiography, we used an argon laser emitting at 488 nm for excitation and filters blocking transmission of wavelengths below 510 nm. For ICG angiography, we used a diode laser emitting at 795 nm and blocking filters for wavelengths below 835 nm. Digital images were recorded for a minimum of 15 minutes after injection and analysed. Imaging software contained in the Heidelberg retinal angiograph was used to identify the same region of comparison for both the fluorescein and ICG angiography images. When analysing the ICG images, we investigated filling delays in the choroidal arteries and choriocapillaris and abnormal choroidal late ICG staining were analysed. In addition, we analysed the small, localised hypofluorescent areas around the leakage points, which were defined as areas without staining during dye passage from the early to the late phase in ICG angiography.
For blood flow measurements, laser Doppler flowmetry was used to assess the relative choroidal blood flow in the centre of the fovea. Briefly, determination of the relative choroidal blood flow was done using a method based on a laser Doppler flowmetry technique, which has been described previously.15,16 A diode laser beam (670 nm) with an intensity of 20 μW was delivered through a fundus camera (Model TRC; Topcon, Tokyo, Japan). The diameter of the probing laser was approximately 200 μm. During measurements, an area of the posterior retina was illuminated at a wavelength of 570 μm with a retinal irradiance of 0.03 mW/cm2, and proper fixation was ascertained by direct observation of the foveola through the fundus camera. All measurements were performed with the subjects seated in a darkened room. Choroidal blood flow measurements were obtained in 22 eyes of 11 patients with CSC (44 (SD 6) years of age). We continuously measured choroidal blood flow in each subject for approximately 30 seconds. The mean value of two choroidal blood flow measurements was calculated in each case. The choroidal blood flow values obtained in eyes with CSC were compared with those of the fellow eyes.
The Mann-Whitney U test was used for statistical analysis to compare choroidal blood flow in CSC eyes and fellow eyes. p Values less than 0.05 were considered to be statistically significant.
RESULTS
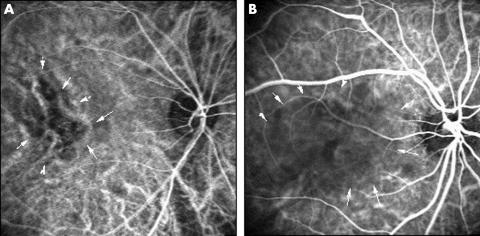
Focal leakage at the level of the RPE was observed in all patients using fluorescein angiography. The early phase ICG angiograms showed filling delays ranging from 1 disc diameter to a larger area with a geographic pattern in the choroidal arteries and choriocapillaris in 27 (75%) of 36 eyes (Fig 1). These filling delays persisted until the dye filled the retinal veins. Focal leakage from the RPE was observed during fluorescein angiography in the areas of the filling delays in all 36 eyes.
Figure 1.
Typical cases of delayed arterial filling using ICG angiography. (A) Right eye of a 52 year old man with CSC. ICG angiography (angle, 30°) 24 seconds after dye injection shows delayed arterial filling in the foveal region (arrows). (B) Right eye of a 41 year old man. Delayed arterial filling is observed 33 seconds after dye injection (arrows) (angle, 30°).
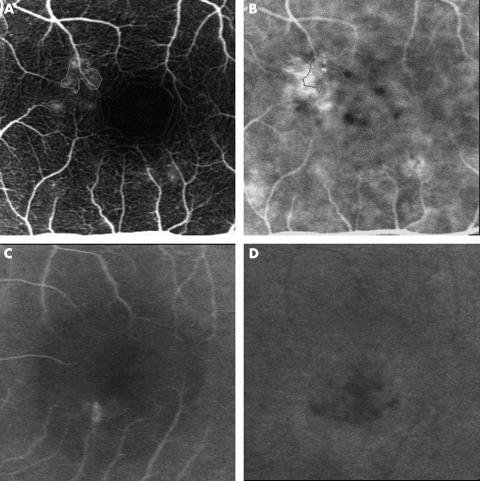
Small, localised hypofluorescent areas around the leakage point detected by fluorescein angiography were obtained during ICG angiography in 27 eyes (75%). Figure 2 shows typical cases of localised hypofluorescent seen during ICG angiography. These hypofluorescent areas were continuously observed throughout dye passage. In these regions biomicroscopy and fluorescein angiography showed no abnormalities such as RPE detachment or atrophy.
Figure 2.
Small, localised hypofluorescent areas around the fluorescein leaking point. (A) The left eye of a 49 year old man with CSC visualised by fluorescein angiography (angle, 10°) 53 seconds after dye injection has fluorescein leakage near the foveal region (thin line). (B) Simultaneous image of ICG angiography. The thin line indicates the same region of fluorescein leakage. Note that the small, localised hypofluorescent areas are seen around the leaking point. (C) Late phase fluorescein angiography (angle, 10°) 10 minutes after dye injection in the right eye of a 32 year old man with CSC shows fluorescein leakage and weak pooling. (D) Simultaneous image of ICG angiography. Localised hypofluorescent areas are seen around the fluorescein leaking point.
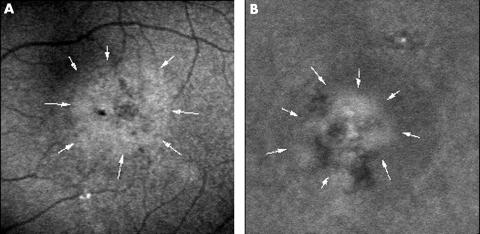
In the late phase angiogram, abnormal choroidal hyperfluorescence was observed in 30 eyes (83%) (Fig 3) when the intravascular dye had been washed out. The sizes of the hyperfluorescent areas ranged from 0.5 to 3.0 disc diameters and they were observed surrounding the leakage point in all 30 eyes and in areas away from the leakage points in 17 (47%) of the 36 eyes.
Figure 3.
Abnormal choroidal hyperfluorescence with ICG angiography. (A) The right eye of a 37 year old man with CSC. ICG angiography (angle, 10°) 14 minutes after dye injection shows hyperfluorescent area about 1.5 disc diameters in size (arrows). (B) The right eye of a 32 year old man visualised during ICG angiography. A hyperfluorescent area with localised hypofluorescence is seen 12 minutes after dye injection (arrows) (angle, 10°).
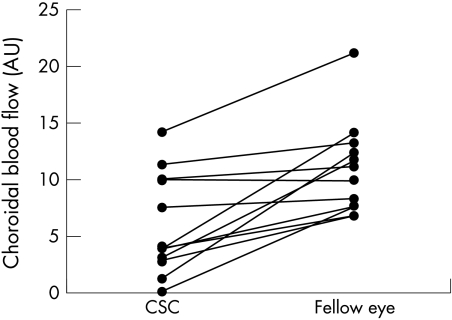
To identify the quantitative state of the choroidal circulation, we measured choroidal blood flow in eyes with CSC and their fellow eyes in 11 patients. The results of the choroidal blood flow measurements are shown in Figure 4. The foveal choroidal blood flow in eyes with CSC (6.27 (4.64) arbitrary units (AU)) was significantly lower (45%) than in fellow eyes (11.41 (4.09) AU) (p<0.01).
Figure 4.
Relative foveolar choroidal blood flow (expressed in AU) in eyes with CSC and fellow eyes.
DISCUSSION
Previously reported findings during ICG angiography of disturbances in the choroidal circulation are filling delays of the choroidal arteries and choriocapillaris,6,10,12 venous dilation,10,12 and focal hyperfluorescence of the choroid.6–12 In the present study, delayed arterial filling was found in 27 eyes (75%). The incidences of this delayed filling vary: 63% reported by Scheider et al,6 71% by Iida et al,12 and 100% by Prunte and Flammer.10 Although the precise aetiology of the delayed filling is unclear, it might be correlated with the evaluation of intravenous pressure in the choriocapillaris or choroidal vein. Prunte and Flammer speculated that delayed arterial filling is caused by capillary and venous congestion, because leakage from the RPE occurred only in an area in which ICG angiography showed choroidal capillary or venous congestion. Our study confirmed the theory, because all fluorescein leakage points from the RPE were also in an area in which ICG angiography disclosed delayed arterial filling.
We also observed focal hyperfluorescence (83%) in the present study, which has been interpreted as regional vascular hyperpermeability.6–12 The incidence of choroidal hyperfluorescence also differs among investigators. Scheider et al,6 Prunte and Flammer,10 and Iida et al12 observed this feature in seven (37%) of 19 eyes, 19 (59%) of 32 eyes, and 101 (96%) of 105 eyes, respectively, using scanning laser ophthalmoscopy. Using a digital ICG fundus camera system, Guyer et al7 and Piccolino et al8,9 reported 100% and 94%, respectively. The differences in the incidences among the studies may depend on different angiographic methodology, such as equipment for ICG angiography and the dose of ICG. Although the findings varied, the results of the present study confirm recent ICG angiographic studies. In our study, we did not investigate dilation of choroidal vasculature because its definition is unclear. However, we also observed dilated vasculature.
In addition to previous ICG angiography findings, we also frequently observed small, localised hypofluorescent areas around the fluorescein leakage. Prunte and Flammer described this finding, but its frequency was unclear.10 Hayashi and coworkers also found similar focal choroidal ischaemia but late hyperfluorescence was observed by fluorescein angiography in their case. In addition, the optical resolution of ICG angiograms was poor at that time and did not permit evaluation of smaller choroidal structures.13 We used simultaneous fluorescein and ICG angiography with confocal scanning laser ophthalmoscopy (Heidelberg retinal angiograph) in the present study, which enabled us to identify the same regions of both fluorescein and ICG angiography.14 Along with the findings obtained using this technique, the hypofluorescent areas disclosed by ICG angiography may be associated with non-perfused choriocapillaris, because continuous hypofluorescence was observed from the early to the late phase of the ICG angiogram. Guyer et al7 reported multiple, “occult,” presumed RPE, detachments in CSC. Multiple RPE detachments were seen as early hyperfluorescence with central hypofluorescence and a ring of hyperfluorescence later in the ICG angiogram in that study. In the present study hyperfluorescence was not seen in the early phase of the ICG angiogram, and we consider our feature to be different from RPE detachment. These localised continuous hypofluorescent areas were observed only around the leakage point detected by fluorescein angiography. We hypothesised that the primary cause of CSC is occlusion of the choriocapillaris, which agrees with the hypothesis of Prunte and Flammer.10 There is supporting evidence that plasminogen activator inhibitor 1, the major antifibrinolytic agent, is increased in patients with CSC.17 Those authors hypothesised that the choroidal circulatory disturbance in CSC is caused by impaired fibrinolysis and the resulting thrombotic occlusion in the choroidal veins.17 On the other hand, it is well known that intravenous injection of adrenaline (epinephrine) that causes vasoconstriction in the venous system18,19 produces a condition similar to CSC in an experimental animal model. Small, localised ischaemic regions caused by non-perfusion or vasoconstriction of the choriocapillaris may induce collateral choriocapillary congestion around this region. The choroidal hyperpermeability resulting from vessel congestion may be observed as localised hyperfluorescence on ICG angiography. Clonic choroidal hyperpermeability may affect to RPE mechanically or metabolically and lead to changes in RPE function, which were previously considered.1,2,4–7,10,17
An additional new finding in our study is decreased foveal choroidal blood flow. Because angiography is a qualitative method, we tried to quantitatively analyse the choroidal circulation. We used a laser Doppler flowmetry technique, the value of which is already established in patients with age related macular degeneration.16 To our knowledge, this is the first study to measure the blood flow in the choriocapillary in CSC. Although bilateral choroidal vascular abnormalities in CSC were reported,12 we compared the choroidal blood flow in eyes with CSC with the blood flow in the fellow eyes in this study. Interestingly, the blood flow in the choriocapillary in eyes with CSC was significantly lower than in the fellow eyes. Although there is little information about this new finding, it might correlate with the non-perfused choriocapillaris observed by ICG angiography. The non-perfused region may lead to and increase choroidal vascular resistant resulting from chronically congested capillaries and/or venous congestion and finally lead to decreased choroidal blood flow. Further research is necessary.
In conclusion, CSC is not merely an RPE disease, but it involves choroidal vascular abnormalities including hyperpermeability and decreased foveal choroidal blood flow. Although the mechanism of these features remains speculative, this choroidal circulatory disturbance may be associated with localised occlusion of the choriocapillaris that was highly obtained during ICG angiography.
Footnotes
The authors have no proprietary interest in any aspect of this study.
REFERENCES
- 1.Gass JDM. Pathogenesis of disciform detachment of the neuroepithelium, II: idiopathic central serous chorioretinopathy. Am J Ophthalmol 1967;63:587–615. [PubMed] [Google Scholar]
- 2.Yannuzzi LA, Gitter KA, Schats H. Central serous chorioretinopathy. In: Yannuzzui LA, Gitter KA, Schats H, eds. The macula: a comprehensive text and atlas. Baltimore: Williams & Wilkins, 1979.
- 3.Yannuzzi LA, Shakin JL, Fisher YL, et al. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology 1984;91:1554–72. [DOI] [PubMed] [Google Scholar]
- 4.Spitznas M. Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol 1986;224:321–4. [DOI] [PubMed] [Google Scholar]
- 5.Marmor MF. New hypothesis on the pathogenesis and treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol 1988;226:548–52. [DOI] [PubMed] [Google Scholar]
- 6.Scheider A, Nasemann JE, Lund OE. Fluorescein and indocyanine green angiographies of central serous choroidopathy by scanning laser ophthalmoscopy. Am J Ophthalmol 1993;115:50–6. [DOI] [PubMed] [Google Scholar]
- 7.Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol 1994;112:1057–62. [DOI] [PubMed] [Google Scholar]
- 8.Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina 1994;14:231–42. [DOI] [PubMed] [Google Scholar]
- 9.Piccolino FC, Borgia L, Zinicola E, et al. Indocyanine green angiographic findings in central serous chorioretinopathy. Eye 1995;9:324–32. [DOI] [PubMed] [Google Scholar]
- 10.Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol 1996;121:26–34. [DOI] [PubMed] [Google Scholar]
- 11.Menchini U, Virgili G, Lanzetta P, et al. Indocyanine green angiography in central serous chorioretinopathy. ICG angiography in CSC. Int Ophthalmol 1997;21:57–69. [DOI] [PubMed] [Google Scholar]
- 12.Iida T, Kishi S, Hagimura N, et al. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina 1999;19:508–12. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol 1986;9:37–41. [DOI] [PubMed] [Google Scholar]
- 14.Freeman WR, Bartsch DU, Mueller AJ, et al. Simultaneous indocyanine green and fluorescein angiography using a confocal scanning laser ophthalmoscope. Arch Ophthalmol 1998;116:455–63. [DOI] [PubMed] [Google Scholar]
- 15.Riva CE, Cranstoun SD, Grunwald JE, et al. Choroidal blood flow in the foveal region of the human ocular fundus. Invest Ophthalmol Vis Sci 1994;35:4273–81. [PubMed] [Google Scholar]
- 16.Grunwald JE, Hariprasad SM, DuPont J, et al. Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci 1998;39:385–90. [PubMed] [Google Scholar]
- 17.Iijima H, Iida T, Murayama K, et al. Plasminogen activator inhibitor 1 in central serous chorioretinopathy. Am J Ophthalmol 1999;127:477–8. [DOI] [PubMed] [Google Scholar]
- 18.Luscher TF, Yang Z, Tschudi M, et al. Interaction between endothelin-1 and endothelium-derived relaxing factor in human arteries and veins. Circ Res 1990;66:1088–94. [DOI] [PubMed] [Google Scholar]
- 19.Haefeli WE, Bargetzi MJ, Starnes HF, et al. Evidence for activation of the sympathetic nervous system by recombinant human interleukin-1 beta in humans. J Immunother 1993;13:136–40. [DOI] [PubMed] [Google Scholar]