Abstract
Aims: To investigate the manifestations and severity of uveitis in children and to identify the risk and specific causes of blindness in this population.
Methods: Retrospective study of data of 123 consecutive patients examined with active uveitis and the onset of ocular disease before the age of 16 years. Numerous variables were assessed including age and sex distribution, laboratory data, the presence of systemic diseases, onset and course of ocular inflammation, clinical features and complications, therapeutic strategies and their outcomes, final visual acuity, and characteristics associated with poor visual outcome.
Results: Systemic disease was observed in 36/123 patients (29%), with juvenile idiopathic arthritis being the most frequent (25/123, 20%). Toxoplasma retinochoroiditis was diagnosed in 12/23 patients with posterior uveitis (52%; 10% of all with uveitis). Severe intraocular inflammation required systemic drugs in 57 (46%) patients. Ocular complications were observed in 93 patients (76%), of which the most common was cataract (43/123, 35%). Intraocular surgery was required in 35 patients (28%; in total 75 procedures). Three patients (2%) became legally blind and an additional 20/121 (17%) had one legally blind eye caused by uveitis. The most frequent causes of blindness were chorioretinal scars in the macular area and glaucoma in contrast with cystoid macular oedema (CMO) in adults.
Conclusions: Uveitis in childhood is a potentially blinding disease, in the majority of patients characterised by a chronic course and a high complication rate.
Keywords: uveitis, childhood, blindness
Intraocular inflammatory disease (uveitis) is a potentially blinding disorder and accounts for 10–15% of all cases of total blindness in United States.1 Legal blindness in at least one eye developed in 22% of all uveitis patients and about 23% of all with required intraocular surgery.2 In the western world, the frequent systemic associations consist of HLA-B27 associated seronegative spondylarthropathies (6%), sarcoidosis (7%), and toxoplasma infection (10%).3 These frequencies are based on a uveitis population of a university referral centre and might vary from a community based uveitis population or throughout the world.4 The uveitis manifests predominantly in young to middle aged adults and approximately 5% of the uveitis population are children younger than 16 years.5–7
Uveitis in childhood differs in various noteworthy aspects from uveitis in adulthood. Firstly, the association with systemic diseases is different in children and ANA positive oligoarticular juvenile idiopathic arthritis (JIA) presents as the most common systemic disorder (41.5%).7,8 Secondly, the use of standard systemic medications such as corticosteroids and immunosuppressive drugs for non-infectious uveitis in childhood has an impact on the immature immune system and developing bones. A growing body of evidence suggests that prolonged administration of corticosteroids is associated with a significant number of permanent adverse effects and steroid sparing drugs are recommended.9–15 Thirdly, the onset of ocular inflammation in children is often asymptomatic despite severe ocular inflammation and with decreased visual acuity already present. Hence, the ocular inflammation is often discovered by routine screening only in the advanced stages of the disease. At the time the child is first seen by an ophthalmologist, ocular complications with negative effects on visual prognosis are regularly detected.16,17 In addition, in children younger than 7 years the risk of amblyopia must be considered. Finally, the results of surgical procedures for the various complications of uveitis (for example, cataract, glaucoma) in children are often discouraging.18–21 The exact visual prognosis of children with JIA is not known; in the past 5 years, rates of blindness ranging from 6–25% have been published for JIA associated uveitis.16,22 A recent review of the literature indicated that up to one third of all children with uveitis ended with severe visual impairment.23 The aim of this study was to investigate the manifestations and severity of uveitis in children and to identify the risk and specific causes of blindness in this population.
PATIENTS AND METHODS
The study included 123 consecutive patients with active uveitis examined at the uveitis clinic of the FC Donders Institute of Ophthalmology of the University Hospital in Utrecht, Netherlands. The selection criterion for childhood uveitis was the onset of uveitis before the age of 16 years. The average follow up was 3 years (range 6 months to 19 years).
We conducted a retrospective analysis of medical records of all patients. Special attention was paid to the relevance of diverse diagnostic procedures, the presence of systemic diseases, the necessity for treatment with systemic immunosuppressive drugs and corticosteroids, ocular complication rates, and visual loss. The patients were classified by the location and specific diagnosis of the uveitis according to the criteria of the International Uveitis Study Group.24 The uveitis was considered chronic if the duration of active ocular inflammation was longer than 3 months. Depending on the severity of the disease and clinical presentation, patients underwent a uveitis screening protocol which encompassed erythrocyte sedimentation rate, complete blood count, serum angiotensin converting enzyme (ACE) level (n=64), and antinuclear antibody (n=70; ANA titre >1:40 was considered positive) tests. HLA-B27 typing was performed in patients with anterior or panuveitis (n=34). In case of suspected infectious uveitis, serological tests were performed for Toxoplasma gondii (n=16), Borrelia burgdorferi (n=18), Bartonella henselae (n=14), Toxocara (n=5), Ascaris (n=6), and Epstein-Barr virus (n=3). Diagnostic aqueous sampling (n=29) was performed according to an earlier protocol and intraocular fluid was analysed for Toxoplasma gondii, herpes simplex virus (HSV), and varicella zoster virus (VZV) (polymerase chain reaction and antibodies).25 Depending on the medical history, clinical presentation, and results of uveitis screening, a subsequent referral to the specialist followed. The diagnosis of JIA was according to the criteria from the International League against Rheumatism (ILAR).26,27 In short, JIA was defined as arthritis starting before the age of 16 years with duration longer than 3 months and the patients had no otherwise identifiable cause for their uveitis.
The diagnosis of JIA was confirmed by a paediatric rheumatologist in all cases. For the diagnosis of sarcoidosis histological examination was required. The clinical diagnosis of ocular toxoplasmosis was based on criteria formulated by Holland et al28; in short, the presence of an active creamy-white focal retinal lesion eventually combined with hyperpigmented retinochoroidal scars in either eye.28
Additional associated diseases were diagnosed according to current standard criteria. All patients underwent a complete ocular examination including measurements of corrected visual acuity, slit lamp examination, funduscopy, and tonometry. Orthoptic examination, fluorescein angiography and visual fields examination were performed in selected cases. Legal blindness was defined as visual acuity ≤0.1 in the better eye and visual impairment as ≤0.3.29 In the visual outcome analysis, only eyes with visual loss due to uveitis were included (excluded were two patients with blindness due to other causes, Leber’s hereditary optic neuropathy, and optic nerve hypoplasia, respectively).
RESULTS
The patient series consisted of 69 girls (56%) and 54 boys (44%). The mean age of onset of uveitis was 8 years with a range of 2–15 years (including one patient with congenital toxoplasma scar without activity and active toxoplasma retinochoroiditis at the age of 11 years). The median age was 7 years. The anatomical location of uveitis was anterior in 44 (36%), intermediate in 30 (24%), posterior in 23 (19%), and panuveitis occurred in 26 patients (21%). The uveitis was chronic in 102/123 patients (83%) and bilateral in 88/123 patients (73%) resulting in 211 affected eyes.
A systemic disease was observed in 36/123 patients (29%); the specific systemic and ocular diagnoses are shown in Table 1. Juvenile idiopathic arthritis was the most common associated systemic disease (25/123, 20%), subdivided into ANA positive oligoarticular JIA (n=22), ANA negative oligoarticular JIA (n=1), oligoarticular JIA (ANA unknown; n=1), and systemic JIA (n=1). Infectious causes were observed in 16 (13%) of the patients. Toxoplasma retinochoroiditis was diagnosed in 12/23 patients with posterior uveitis (52%; 10% of all with uveitis). Four cases of anterior uveitis were caused by herpes viruses. Histologically proved sarcoidosis was diagnosed in three patients (all with extrapulmonary locations; joints and skin in two cases and renal manifestation in one). Ocular features of all these patients included peripheral multifocal choroiditis with vasculitis (Fig 1). Unexpected diagnosis of multiple sclerosis was made in a 14 year old boy who presented with panuveitis at the age of 6 years. His diagnosis was delayed because of non-specific neurological symptoms and a borderline positive ANA titre. In his case, the diagnosis was based on cerebral magnetic resonance imaging (MRI) and brain biopsy in the absence of oligoclonal bands in spinal fluid. Another 11 year old girl with panuveitis was considered to have presumed multiple sclerosis, since demyelinating plaques were noted on the MRI scan of the brain. So far, no neurological symptoms or cerebrospinal fluid abnormalities have developed. Ocular features in both children included papillitis with cystoid macular oedema (CMO), peripheral vasculitis, and vitreous opacities. Both children were repeatedly negative in various diagnostic tests for sarcoidosis, Lyme borreliosis and other disorders, which could otherwise explain the combination of central nervous system and ocular involvement. A 6 year old girl with masquerade syndrome (unilateral hypopyon uveitis) was ultimately diagnosed with retinoblastoma. In none of the patients with intermediate uveitis was a systemic disease disclosed.
Table 1.
Diagnosis and associated systemic diseases according to anatomic uveitis entities
| Systemic disease or specific ocular diagnosis | No of patients | No of patients with anterior uveitis | No of patients with intermediate uveitis | No of patients with posterior uveitis | No of patients with panuveitis |
| Systemic disease: | |||||
| JIA | 25 | 18 | 7 | ||
| Sarcoidosis | 3 | 3 | |||
| TINU syndrome | 2 | 1 | 1 | ||
| CINCA | 3 | 2 | 1 | ||
| Psoriasis | 1 | 1 | |||
| Multiple sclerosis | 1 | 1 | |||
| Masquerade syndrome | 1 | 1 | |||
| Infections: | |||||
| Toxoplasmosis | 12 | 12 | |||
| Herpesviruses | 4 | 4 | |||
| Specific ocular disease: | |||||
| Fuchs | 1 | 1 | |||
| AMPPE | 1 | 1 | |||
| DUSN | 1 | 1 | |||
| Phacogenic uveitis | 1 | 1 | |||
| Trauma | 1 | 1 | |||
| Unknown | 66 | 18 | 30 | 7 | 11 |
| Total | 123 | 44 | 30 | 23 | 26 |
JIA = juvenile idiopatic arthritis; TINU = tubulointerstitial nephritis and uveitis; CINCA = chronic infantile neurological cutaneous and articular/neonatal onset multisystem inflammatory disease syndrome; AMPPE = acute multifocal placoid pigment epitheliopathy; DUSN = diffuse unilateral subacute neuroretinitis.
Figure 1.
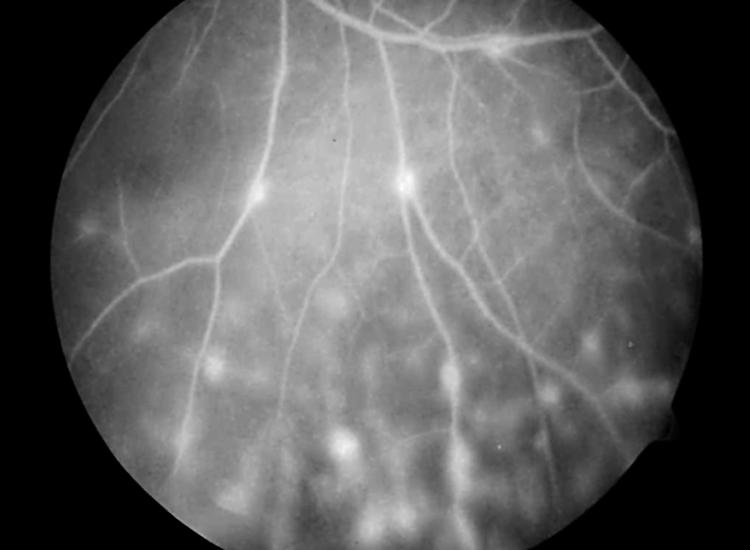
Peripheral segmental vasculitis and periphlebitis in an 8 year old boy with sarcoidosis.
Antinuclear antibodies were positive in 27 of the 70 (39%) patients tested and 22 of the 27 (82%) ANA positive patients suffered from JIA (12 girls and 10 boys). A 5 year old patient diagnosed with oligoarticular JIA and uveitis was repeatedly ANA negative. The remaining five ANA positive patients, all girls, had uveitis clinically consistent with uveitis occurring with JIA (one also with arthritis) but did not fulfil the diagnostic criteria of JIA. HLA-B27 was positive in 3/34 (9%) patients tested, but none of them suffered from acute anterior uveitis.
ACE was elevated in 19/64 (30%) patients tested, of which one had biopsy confirmed sarcoidosis (ACE level 44 U/l; cut-off level in our laboratory is 20U/l). The ACE levels in two remaining patients with sarcoidosis were within normal limits, but these were tested with corticosteroids or immunosuppressive medication. The remaining elevated serum ACE levels (22–31 U/l) were observed in anterior uveitis of unknown origin (n=6), Fuchs’ heterochromic cyclitis (n=1), intermediate uveitis (n=5), posterior uveitis of unknown origin (n=2), panuveitis (n=3), and tubulointerstitial nephritis and uveitis (TINU syndrome (n=1)).
Aqueous humour analysis revealed a specific diagnosis in 5/29 (17%) patients tested. Specifically, active intraocular antibody production against varicella zoster virus was detected in 2/6 patients with anterior uveitis and Toxoplasma gondii in 3/6 cases with posterior uveitis tested. Aqueous humour analysis was negative in patients with intermediate uveitis (n=10) and panuveitis (n=7) tested.
Complications of uveitis developed in 93/123 (76%) of the patients (Table 2). The most frequent complications were cataract (43/123, 35%) and glaucoma (23/123, 19%; 48% of them with JIA). Optic disc oedema was noted in 36/123 (29%) patients (funduscopy, n=11; fluorescein angiography, n=25).CMO was detected in 21/123 (17%) patients. Intraocular surgery was required in 35/123 (28%).
Table 2.
Complications of uveitis in children
| Complications* | No | % |
| Cataract | 43 | (35%) |
| Glaucoma | 23 | (19%) |
| Optic disc leakage/oedema† | 36 | (29%)† |
| Cystoid macular oedema | 21 | (17%) |
| Macular scar | 8 | (7%) |
| Hypotony | 4 | (3%) |
| Retinal detachment | 3 | (2%) |
| Vitreous haemorrage | 2 | (2%) |
| Band kerathopathy | 15 | (12%) |
| Corneal opacities | 4 | (3%) |
| Iris atrophy | 2 | (2%) |
| Strabisms | 5 | (4%) |
| Miscellaneous‡ | 3 | (3%) |
*The patients can have more than one complication. †In 25 patients, the papilloedema or leakage was diagnosed by fluorescein angiography. ‡miscellaneous includes macular pucker, subretinal neovascularisation and retinal hole.
The visual outcome is shown in Table 3. Three patients (2%) fulfilled the criteria of bilateral legal blindness. Their diagnoses included ANA positive JIA associated uveitis, multiple sclerosis, and macular scars of unknown origin. Unilateral blindness was documented in 20/121 (17%) patients (JIA associated uveitis (n=4), toxoplasmosis (n=4), pars planitis (n=4), diffuse unilateral subacute neuroretinitis (n=1), herpetic uveitis (n=1), masquerade syndrome (n=1), and unknown origin (n=5)). Eight patients (7%) had unilateral visual impairment (visual acuity ≤0.3).
Table 3.
Visual outcome of children with uveitis
| No of patients | ||||
| Bilateral | Unilateral | |||
| Visual acuity | No | % | No | % |
| ≤20/200 | 3 | 2 | 20 | 17 |
| 0.1 ≤0.3 | 0 | 0 | 8 | 7 |
| 0.3 ≤0.5 | 3 | 2 | 11 | 9 |
| >0.5 | 76 | 63 | ||
| Total | 6 | 4 | 115 | 96 |
The ocular complications responsible for legal blindness (VA ≤0.1) are given in Table 4. The most frequent causes of blindness were macular scars (seven eyes, due to toxoplasma retinochorioiditis in four cases) and secondary glaucoma (n=4; three with JIA and one with intermediate uveitis). The most frequent cause of visual impairment was CMO (n=3). One patient with blindness due to uveitis lived in a blind institute and 11 patients (9%) received special education programmes because of their visual handicap.
Table 4.
Ocular complications leading to visual acuity of less than 20/200
| Cause of visual loss | No of eyes | % |
| Macular scar of toxoplasmic origin | 4 | 15 |
| Macular scars of unknown origin | 3 | 12 |
| Secondary glaucoma | 4 | 15 |
| Cystoid macular oedema | 2 | 8 |
| Optic neuritis | 2* | 8 |
| Hypotony/atrophy | 2 | 8 |
| Retinal detachment | 2† | 8 |
| Miscellaneous | 7‡ | 27 |
| Total | 26 | 100 |
*Bilateral blindness in a patient with MS; †exudative detachment in one case; ‡miscellaneous includes choroidal detachment, vitreous haemorrhage, vitreous opacities, subretinal neovascularisation, enucleation, corneal opacities, and mature cataract.
Systemic treatment was required in 57 patients (46%) to control intraocular inflammation (Table 5). Prednisolone (n=39, 32%) was the most commonly used anti-inflammatory drug and was used either in combination with other immunosuppressive medications (n=7) or as an adjuvant in treatment for infectious uveitis (n=10). Methotrexate (MTX) was given in 11 cases, the majority of those with JIA associated uveitis (dose 8–12 mg per m2). In three (27%) of these patients, the MTX dosage had to be reduced because of elevated liver enzymes (reversible in all). Treatment with NSAIDs (n=16; 13%) was applied for JIA associated uveitis (n=14), sclerouveitis (n=1), and intermediate uveitis (n=1). In eight cases prolonged acetazolamide therapy was prescribed (glaucoma in three cases, CMO in four cases, or both in one case). Specific anti-infectious medication was used in 10 cases. Three patients were treated with salazopyrine and one patient with anti-TNF receptor for their rheumatic disease. In 41 cases (33%) subconjunctival corticosteroid injections were used and corticosteroid eye drops in 107 patients (87%).
Table 5.
Medical treatment for paediatric uveitis
| Medication* | No of patients | % |
| Systemic: | 57 | 46 |
| Immunosuppressive | ||
| Corticosteroids | 39 | 32 |
| Methotrexate | 11 | 9 |
| Cyclosporin | 4 | 3 |
| Anti-inflammatory | ||
| NSAID | 16 | 13 |
| Anti-infectious | ||
| Anti-toxoplasma | 7 | 6 |
| Anti-herpesvirus | 2 | 2 |
| Antibiotics | 1 | 1 |
| Other | ||
| Acetazolamide | 8 | 7 |
| Periocular corticosteroid injections | 41 | 33 |
| Corticosteroid eye drops | 107 | 87 |
*One patient can be treated with multiple drugs.
Thirty five patients (28%) underwent surgery for complications of uveitis. In total, 75 operations were performed with a maximum of six operations per patient (Table 6). Cataract surgery was the most commonly performed operation (34 eyes of 23 patients; 18 eyes with anterior vitrectomy and 15 eyes with an intraocular lens).Trabeculectomy was performed in eight patients (nine eyes; five eyes with mitomycin, two eyes with 5-fluorouracil, and two eyes without cytostatics; one eye was operated twice). Failure of primary trabeculectomy occurred in four patients (44%; one of them without cytostatics).
Table 6.
Surgical procedures performed because of complications of uveitis in children
| Surgical procedure | No of operations |
| Cataract extraction | 34 |
| Trabeculectomy | 10 |
| Pars plana vitrectomy | 11 |
| Cryocoagulation peripheral retina | 8 |
| Capsulotomy | 3 |
| EDTA chelation | 2 |
| Sector or peripheral iridectomy | 2 |
| Miscellaneous* | 5 |
| Total | 75 |
*Miscellaneous includes enucleation, conventional retinal detachment surgery, pupil reconstruction, removal of corticosteroid depot, and strabismus.
DISCUSSION
This study points out that uveitis of childhood is a very serious and potentially blinding disease. The high complication rate of 76% and the need for systemic treatment in almost half of the cases emphasises the severity and chronicity of uveitis at a young age. The follow up of occasional patients is relatively short (6 months) and the likelihood of developing complications like glaucoma during this period is low. This points out that even after the average follow up limited to the 3 years, 19% of young patients with uveitis will develop at least one legally blind eye. This percentage might even become higher with longer follow up time. Similar rates of blindness were found in an adult uveitis population.2 However, the principal causes of visual loss in uveitis of children were clearly distinct from an adult population: specifically macular chorioretinal scars and secondary glaucoma in children in contrast with CMO observed in adults. The macular scars, which constituted a major cause of blindness in childhood uveitis, were predominantly caused by ocular toxoplasmosis, the most common infectious cause of posterior uveitis. Active prevention of infections with Toxoplasma gondii, congenital as well as acquired, might help to reduce blindness of childhood uveitis in the future.
The second cause of blindness in childhood uveitis was secondary glaucoma, the prognosis of which was earlier noted to be poor. Kanski et al reported that one third of the glaucomatous eyes in JIA ended with no light perception and attributed the poor prognosis to difficult diagnosis in uncooperative young patients, unsatisfactory therapy, and low success rate of conventional filtration surgery (18%).17 So far, no consensus exists for a primary surgical procedure for secondary glaucoma in childhood; specifically it is not clear whether to use mitomycin, 5-fluoruracil or drainage implants.30,31 The policy of our institute was to treat children with secondary glaucoma resistant to maximal topical therapy with long term carbonic anhydrase inhibitors. We attempted to postpone the surgery until an older age, or at least until the uveitis was quiet for at least 6 months. However, we did operate on children with progressive visual field loss despite the above treatment and used trabeculectomy with or without application of local cytostatics under the cover of maximal anti-inflammatory therapy as a primary procedure. In our series, failure of primary trabeculectomy occurred in four patients (44%).
Cystoid macular oedema complicated uveitis less frequently in children (17%) compared with adults (26%) and was the cause of legal blindness in only 8% of children compared to 29% of adults with uveitis (the most frequent cause of blindness and visual impairment in an adult uveitis population).2 Hypothetical explanations could also include the strong vitreoretinal adherence in youth, which might prevent the development of CMO. Support for this hypothesis might be that CMO rarely develops after cataract surgery with removal of the posterior capsule in non-uveitic eyes in childhood.32–36 However, the low frequency of CMO and its limited visual loss in young patients needs further investigation.
According to other studies, JIA was the most common associated systemic disease in the paediatric uveitis population.7,8 It must be stressed that uveitis may develop years after the onset of JIA (8% in our JIA associated uveitis population). Therefore, repeated ophthalmological evaluations should be periodically performed according to the recommendations of the American Academy of Pediatrics Section on Rheumatology and Section on Ophthalmology.37
Various diseases associated with uveitis in childhood may strongly resemble JIA. Firstly, sarcoidosis of childhood frequently manifests in the joints, skin, and eyes and rarely in the lungs.38 Posterior segment manifestations (retinal vasculitis, peripheral multifocal chorioiditis, and choroidal granuloma) observed by the ophthalmologist might be decisive in the diagnostic process.39 Furthermore, systemic diseases with similarities with JIA is the CINCA/NOMID syndrome.40–42 This is a rare syndrome characterised by arthritis and anterior uveitis. The absence of posterior synechiae formation and no tendency for cataract formation are characteristic.41 Furthermore, in 83% of the patients with CINCA syndrome, papillitis is observed, whereas this is only seen in 6% of the patients with uveitis in JIA.8 Our results are representative for a tertiary referral centre and might explain the relatively high number of patients with CINCA syndrome and other systemic diseases.
In our series, infectious uveitis was noted in at least 13% of children. Aqueous humour analysis specifically for toxoplasmosis or herpes viruses was beneficial in diagnosing intraocular infections.25 Although positive serological results might be more informative in young children than in adults, positive antibodies are not discriminatory for intraocular infection. Rare causes of uveitis included masquerade syndrome in a 6 year old girl who appeared to have a retinoblastoma. Previously, 8% of the children with retinoblastoma were primarily diagnosed with uveitis and though rare, the possibility of masquerade syndrome must be considered, especially in the very young.43
The value of positive ANA is very useful in suspicious diagnosis of JIA. However, it should be noted that this test is non-specific and might indicate the presence of other diseases including systemic lupus erythematosus or multiple sclerosis.44–46 Kimura first reported positive ANA in uveitis in young girls without JIA6 and also, in our series, the patients with a positive ANA reaction, who did not fulfil the criteria of JIA, were all female. Serum ACE levels are frequently raised in healthy children (in our uveitic population 30%). Although the raised serum ACE levels were reported to be of diagnostic importance in adults with uveitis, their value in childhood population is limited but47 in view of our results and earlier recommendations we selected a very limited screening protocol for uveitis of childhood (Table 7). Although erythrocyte sedimentation rate, red and white blood cell count, glucose, syphilis serology, and chest radiography were recommended in adults,3 we decided not to include these tests in the initial screening in children, but to perform them only in selected cases. Despite aggressive systemic treatment, 19% of our patients developed at least one blind eye. The new therapeutic strategies, such as the use of MTX, were reported to improve the control of intraocular inflammation and have beneficial visual results especially in JIA associated cases.48–50 Compared to other anti-inflammatory medications, MTX was well tolerated in childhood14 and has no cataractogenic or eye pressure elevating properties. The eventual ophthalmological benefit of new anti-inflammatory drugs in JIA as anti-TNF-α,51,52 leflunamide,53 or even stem cell transplantation54 was not yet evaluated.
Table 7.
Initial screening for uveitis in childhood (<16 year of age)
| Anterior uveitis | Intermediate and posterior uveitis | Panuveitis | |
| Antinuclear antibodies | + | – | + |
| Angiotensin converting enzyme | + | + | + |
| HLA-B27 | + | – | + |
| Aqueous humour analysis* | + | + | + |
*In cases of presumed infectious uveitis.
Our study points out that uveitis of childhood is a potentially blinding disease associated with a complication rate of 76% and surgical intervention in 28%. Blind eyes developed in 19% of patients and macular scars and secondary glaucoma were the major causes of visual loss. Early diagnosis, timely recognition, or prevention of complications with new treatment strategies will hopefully improve the visual prognosis of children with uveitis.
REFERENCES
- 1.Nussenblatt RB. The natural course of uveitis. Int Ophthalmol 1990;141:303–8. [DOI] [PubMed] [Google Scholar]
- 2.Rothova A, Suttorp-Schulten MSA, Treffers WF, et al. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80:332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothova A, Buitenhuis HJ, Meenken C, et al. Uveitis and systemic disease. Br J Ophthalmol 1992;76:137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCannel CA, Holland G N, Helm CJ, et al. Causes of uveitis in the general practice of ophthalmology. Am J Ophthalmol 1996;121:35–46. [DOI] [PubMed] [Google Scholar]
- 5.Perkins ES. Patterns of uveitis in children. Br J Ophthalmol 1966;50:169–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura SJ, Hogan MJ, Thygeson P. Uveitis in children. Arch Ophthalmol 1954;51:80–8. [DOI] [PubMed] [Google Scholar]
- 7.Kanski JJ, Shun-Shin A. Systematic uveitis syndromes in childhood; an analysis of 340 cases. Ophthalmology 1984;91:1247–52. [DOI] [PubMed] [Google Scholar]
- 8.Tugal-Tutkun I, Harvlikova K, Power WJ, et al. Changing patterns in uveitis of childhood. Ophthalmology 1996;103:375–83. [DOI] [PubMed] [Google Scholar]
- 9.Tamesis RR, Rodriguez A, Christen WG, et al. Systemic drug toxicity trends in immunosuppressive therapy of immune and inflammatory ocular disease. Ophthalmology 1996;103:768–75. [DOI] [PubMed] [Google Scholar]
- 10.Santamaria JII. Steroidal agents : their systemic and ocular complication. Ocular Inflamm Ther 1983;1:19–26. [Google Scholar]
- 11.Kilmartin DJ, Forrester JV, Dick AD. Cyclosporin A therapy in refractory non-infectious childhood uveitis. Br J Ophthalmol 1998;82:737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemady RK, Baer JC, Foster CS. Immunosuppressive drugs in the management of progressive corticosteroid-resistant uveitis associated with juvenile rheumatoid arthritis. Int Ophthalmol Clin 1992;32:241–52. [DOI] [PubMed] [Google Scholar]
- 13.Ramadan A, Nussenblatt R. Cytotoxic agents in ocular inflammation. Ophthalmol Clin North Am 1997;10:337–87. [Google Scholar]
- 14.Giannini EH, Brewer EJ, Kuzmina N, et al. Methotrexate in resistant juvenile rheumatoid arthritis. N Engl J Med 1992;326:1043–9. [DOI] [PubMed] [Google Scholar]
- 15.Gerloni V, Cimaz R, Gattinara M. Efficacy and safety profile of cyclosporin A in the treatment of juvenile chronic (idiopathic) arthritis. Results of a 10-year prospective study. Rheumatology 2001;40:907–13. [DOI] [PubMed] [Google Scholar]
- 16.Dana MR, Merayo-Lloves J, Schaumberg DA, et al. Visual outcomes prognosticators in juvenile rheumatoid arthritis-associated uveitis. Ophthalmology 1997; 104:236–44. [DOI] [PubMed] [Google Scholar]
- 17.Kanski JJ. Juvenile arthritis and uveitis. Surv Ophthalmol 1990;34:253–67. [DOI] [PubMed] [Google Scholar]
- 18.Hooper PL, Rao NA, Smith RE. Cataract extraction in uveitis patients. Surv Ophthalmol 1990;35:120–44. [DOI] [PubMed] [Google Scholar]
- 19.Wolf MD, Lichter PR, Ragsdale CG. Prognostic factors in the uveitis of juvenile rheumatoid arthritis. Ophthalmology 1987;94:1242–8. [DOI] [PubMed] [Google Scholar]
- 20.Kanski JJ. Lensectomy for complicated cataract in juvenile chronic iridoclyclitis. Br J Ophthalmol 1992;76:72–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox JGM, Flynn HW, Davis Jr JL, et al. Causes of reduced visual acuity on long-term follow-up after cataract extraction in patients with uveitis and juvenile rheumatoid arthritis. Am J Ophthalmol 1992;114:708–14. [DOI] [PubMed] [Google Scholar]
- 22.Edelsten C, Lee V, Bentley CR, et al. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. Br J Ophthalmol 2002;86:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham ET Jr. Uveitis in children. Ocular Immunol Inflamm 2000;8:251–61. [DOI] [PubMed] [Google Scholar]
- 24.Bloch-Michel E, Nussenblatt RB. International uveitis study group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol 1987;103:234–5. [DOI] [PubMed] [Google Scholar]
- 25.Boer JH de, Verhagen C, Bruinenberg M, et al. Serological and polymerase chain reaction analysis of intraocular fluids in the diagnosis of infectious uveitis. Am J Ophthalmol 1996;121:650–8. [DOI] [PubMed] [Google Scholar]
- 26.Berntson L, Fasth A, Andersson-Gare B, et al. Construct validity of ILAR and EULAR criteria in juvenile idiopathic arthritis: a population based incidence study from the Nordic countries. International League of Associations for Rheumatology. European League Against Rheumatism. J Rheumatol 2001;28:2737–43. [PubMed] [Google Scholar]
- 27.Krumrey-Langkammerer M, Hafner R. Evaluation of the ILAR criteria for juvenile idiopathic arthritis.J Rheumatol 2001;28:2544–7. [PubMed] [Google Scholar]
- 28.Holland GN, O’Connor R, Belfort R, et al. In: Pepose JS, Holland GN, Wilhelmus KR, eds. Ocular Infection and Immunity. St Louis: Mosby-Year Book, 1996:1183–223.
- 29.Kraut JA, McCabe CP. The problem of low vision. Definition and common problems. In: Albert DM, Jakobiec FA, eds. Principles and practice of ophthalmology. Philadelphia: WB Saunders, 1994;5:3664–6.
- 30.Foster CS, Havlrikova K, Baltatzis S, et al. Secundary glaucoma in patients with juvenile rheumatoid arthritis-associated iridocyclitis. Acta Ophthalmol Scand 2000;78:576–9. [DOI] [PubMed] [Google Scholar]
- 31.Välimäki J, Airaksinen J, Tuulonen A. Molteno implantation for secondary glaucoma in juvenile rheumatoid arthritis. Arch Ophthalmol 1997;115:1253–6. [DOI] [PubMed] [Google Scholar]
- 32.Pinchoff BS, Ellis FD, Helveston EM, et al. Cystoid macular edema in pediatric aphakia. J Pediatr Ophthalmol Strabismus 1988;25:240–3. [DOI] [PubMed] [Google Scholar]
- 33.Rao SK, Ravishankar K, Sitalakshmi G, et al. Cystoid macular edema after pediatric intraocular lens implantation: fluorescein angioscopy results and literature review. J Cataract Refract Surg 2001;27:432–6. [DOI] [PubMed] [Google Scholar]
- 34.Morgan KS, Franklin RM. Oral fluorescein angioscopy in aphakic children. J Pediatr Ophthalmol Strabismus 1984;21:33–6. [DOI] [PubMed] [Google Scholar]
- 35.Schulman J, Peyman GA, Raichand M, et al. Aphakic cystoid macular edema in children after vitrectomy for anterior segment injuries. Ophthalmic Surg 1983;14:848–51. [PubMed] [Google Scholar]
- 36.Poer DV, Helveston EM, Ellis FD. Aphakic cystoid macular edema in children. Arch Ophthalmol 1981;99:249–52. [DOI] [PubMed] [Google Scholar]
- 37.American Academy of Pediatrics Section on Rheumatology and Section on Ophthalmology. Guidelines for ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics 1993;92:295–6. [PubMed] [Google Scholar]
- 38.Fink CW, Cimaz R. Early onset sarcoidosis: not a benign disease. J Rheumatol 1977;24:174–7. [PubMed] [Google Scholar]
- 39.Okada AO, Foster CS. Posterior uveitis in the pediatric population. Int Ophthalmol Clin 1992;32:121–52. [DOI] [PubMed] [Google Scholar]
- 40.Sadiq S, Gregson RMC, Downes RN. The CINCA syndrome: a rare cause of uveitis in childhood. J Pediatr Ophthalmol Strabismus 1996;33:59–64. [DOI] [PubMed] [Google Scholar]
- 41.Dolffus H, Hafner R, Hofmann HM, et al. Chronic infantile neurological cutaneous and articular/neonatal onset multisystem inflammatory disease syndrome. Arch Ophthalmol 2000;118:1386–92. [DOI] [PubMed] [Google Scholar]
- 42.Prieur A. Chronic infantile neurological cutaneous and articular/neonatal onset multisystem inflammatory disease syndrome. Arch Ophthalmol 2000;118:1386–92. [DOI] [PubMed] [Google Scholar]
- 43.Stafford WR, Yanoff M, Parnell BL. Retinoblastomas initially misdiagnosed as primary ocular inflammations. Arch Ophthalmol 1969;82:771–3. [DOI] [PubMed] [Google Scholar]
- 44.Vaile JH, Dyke L, Kherani R, et al. Is high titre ANA specific for connective tissue disease? Clin Exp Rheumatol 2000;18:433–8. [PubMed] [Google Scholar]
- 45.Perilloux BC, Shetty AK, Leiva LE, et al. Antinuclear antibody (ANA) and ANA profile tests in children with autoimmune disorders: a retrospective study. Clin Rheumatol 2000;19:200–3. [DOI] [PubMed] [Google Scholar]
- 46.Pelletier J, Ali Cherif A. Multiple sclerosis plus”: leukoencephalopathies at the frontiers of internal. Rev Med Interne 2000;21:1104–13. [DOI] [PubMed] [Google Scholar]
- 47.Baarsma GS, La Hey E, Glassius, et al. The predictive value of serum angiotensin converting enzyme and lysozyme levels in the diagnosis of ocular sarcoidosis. Am J Ophthalmol 1987;104:211–7. [DOI] [PubMed] [Google Scholar]
- 48.Samson CM, Waheed N, Baltatzis S, et al. Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology 2001;108:1134–9. [DOI] [PubMed] [Google Scholar]
- 49.Weiss AH, Wallace CA, Sherry DD. Methotrexate for resistant chronic uveitis in children with juvenile rheumatoid arthritis. J Pediatr 1998;133:266–8. [DOI] [PubMed] [Google Scholar]
- 50.Rose CD, Singsen BH, Eichenfeld AH, et al. Safety and efficacy of methotrexate therapy for juvenile rheumatoid arthritis. J Pediatr 1990;117:653–9. [DOI] [PubMed] [Google Scholar]
- 51.Smith JR, Levinson RD, Holland GN, et al. Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Rheum 2001;45:252–7. [DOI] [PubMed] [Google Scholar]
- 52.Reiff A, Takei S, Sadeghi S, et al. Etanercept therapy in children with treatment-resistant uveitis. Arthritis Rheum 2001;44:1411–15. [DOI] [PubMed] [Google Scholar]
- 53.Boumpas DT, Kritikos HD, Daskalakis NG. Perspective on future therapy of vasculitis. Curr Rheumatol Rep 2000;2:423–9. [DOI] [PubMed] [Google Scholar]
- 54.Wulffraat N, van Royen A, Bierings M, et al. Autologous haemopoietic stem-cell transplantation in four patients with refractory juvenile chronic arthritis. Lancet 1999;35:550–3. [DOI] [PubMed] [Google Scholar]


