Abstract
Aim: To investigate the relative sensitivity and specificity of two tests of retinal function (the electro-oculogram (EOG) and a computerised colour vision test) in screening for ocular toxicity caused by chloroquine and hydroxychloroquine.
Methods: 93 patients with rheumatic diseases receiving long term chloroquine and hydroxychloroquine therapy were followed for an average of 2.6 years. Clinical examination, an EOG, and a quantitative test of colour vision were carried out every 6 months.
Results: Mild fundus changes were observed in 38 patients. Four patients developed typical bull’s eye maculopathy, three of whom had received 250, 365, and 550 g total dose of chloroquine, and one 1500 g of hydroxychloroquine. Statistical analysis of all patients showed that for those with no fundus changes or stippled pigmentation a number showed elevation of tritan threshold, so that if macular stippling is a sign of mild retinopathy the test on tritan changes has a 64% sensitivity and 63% specificity for an upper threshold value of 7%. All four patients with bull’s eye lesions showed a marked disturbance of tritan colour vision, with a threshold of 14.8%, a sensitivity of 75%, and a specificity of 94%. For protan colour vision a threshold of 10% gives 75% sensitivity and 91% specificity. By contrast, neither an absolute nor a relative EOG reduction was a valid criterion for early or late chloroquine retinopathy. In advanced retinopathy an Arden coefficient (AQ) <180% yields 50% sensitivity and 54% specificity. When AQ <160% is the threshold, sensitivity does not increase but specificity rises to 82%. Occurrence of marked corneal deposits on clinical examination yields 50% sensitivity and 90% specificity in this situation.
Conclusion: Screening for chloroquine retinopathy can be improved by using a sensitive colour test. Disturbance of the tritan axis appears to occur first. A normal test result on computerised colour testing virtually excludes any retinopathy by antimalarials. The EOG is of little diagnostic value.
Keywords: antimalarial toxicity, screening, bull’s eye maculopathy, computer colour vision test, electro-oculogram
Ocular toxicity caused by antimalarials was first described in the literature as early as 1957.1,2 As antimalarials were found effective not only for treatment and prophylaxis of malaria but also for many rheumatoid diseases they are used ever more frequently nowadays and are given as a long term medication. The risk of ocular toxicity is therefore considerable. The incidence of early retinopathy in ophthalmologically unmonitored patients was estimated in an early review by Bernstein3 as 10% for chloroquine and 3–4% for hydroxychloroquine. Advanced retinopathy had an incidence of 0.5%. These risks might be reduced substantially by regular observation and testing.
Some degree of corneal deposits (verticillata) can be demonstrated in most patients taking chloroquine, but these changes very rarely impair vision.4 Corneal deposits occur more frequently with chloroquine than with hydroxychloroquine,5 are located in the epithelium and subepithelial stroma6,7 and are mostly reversible.7 By contrast, retinopathy is a severe ocular side effect presenting with bilateral, reproducible, and permanent visual field abnormalities.8 Early retinal changes consist of a pigmentary stippling or granular appearance of the macula, with the patient still being asymptomatic. Advanced retinopathy may show the typical “bull’s eye” maculopathy associated with impaired visual acuity and central visual field defects.9 These changes of advanced maculopathy are irreversible and may progress even after cessation of the drug.10,11
Screening strategies for antimalarial toxicity are still controversial, because there is a difficulty in defining the early stages of retinopathy, and in well monitored patients, the incidence of retinopathy is low. Macular changes and transient defects in visual field testing as well as in colour vision testing may be symptoms of a “pre-retinopathy,” but are difficult to differentiate from other causes such as age related changes. While all screening strategies include visual acuity testing and a dilated fundus examination, and many include fundus photographs, other tests like fluorescein angiography12 or more frequently visual field tests ranging from Amsler grid13 to automated macular perimetry14–16 have been used. Also, various colour vision tests have been proposed17 and different electrophysiological tests (EOG and ERG) have been evaluated.18–25 The efficiency of all such ophthalmological screening procedures is controversial. This is reflected in surveys of current practice patterns showing quite inhomogeneous results.26 Recently, proposed screening requirements are more and more limited to clinical tests,16,27 and for hydroxychloroquine no routine ophthalmological screening is recommended by the Royal College of Ophthalmologists.9 The recent recommendations of the American Academy of Ophthalmology16 adopt screening intervals after a baseline examination for both antimalarials because of the anticipated risk to the patient. The proposed routine screening includes an ophthalmological examination and visual field testing by an Amsler grid or Humphrey 10-2. Optional tests include colour testing, fundus photography and fluorescein angiography, and multifocal ERG. The EOG is not recommended by the American Academy as a screening test.
Therefore the aim of the current study was to investigate, if screening for ocular toxicity of antimalarials can be improved by adding the EOG—as it theoretically shows retinal pigment epithelium and rod/cone interactions—and by colour vision testing. For colour vision testing a computerised test developed by Arden,28 known to be more sensitive than most other colour tests28–30 was applied.
METHODS
Retinopathy
The definition of retinopathy caused by antimalarials is controversial. Subtle retinal changes such as pigmentary stippling or granular appearance of the macula with the patient still being asymptomatic (and not exhibiting defects in visual field testing) may be the first signs of retinopathy.31,32 In those cases colour vision is known to be already reduced.17,31,33 In established retinopathy bilateral, reproducible, and permanent visual field abnormalities are found additionally.8 In more advanced cases also visual acuity may be impaired and the typical “bull’s eye” maculopathy can be seen.9
Therefore in this study two forms of retinopathy were distinguished. A “mild form” of retinopathy was defined by pigmentary macular changes but without reproducible defects on Amsler and static macular visual field testing (Humphrey, 10-2 program). A second group of patients with “advanced” maculopathy distinguished in accordance with previous use8 showed advanced macular changes—that is, typical bull’s eye appearance and reproducible, bilateral visual field defects.
Patients
Retrospectively all patients monitored between 1983 and 2000 at our electrophysiological department for antimalarial toxicity were identified from the records. Inclusion criteria were at least one complete examination for toxicity including clinical examination, EOG, and colour vision testing. Patients with other causes for macular changes such diabetes, central serous retinopathy, retinitis pigmentosa, or age related macula degeneration were excluded. Therefore, from 98 identified patients, five with age related macula degeneration were excluded. In those five patients three had normal EOGs and colour vision, one exhibited only a disturbance in tritan axis, and one patient had an abnormal EOG <160% Arden coefficient and a defect in the tritan axis. Also, patients with other ocular disorders interfering with colour vision such as advanced cataracts, or diseases affecting the optic nerve such as former optic neuritis or glaucoma were excluded. None of the remaining 93 patients had any of these disorders.
A final total of 93 patients were included in the study. Of those, 66 (71%) were female and 27 male. The reason for medication with antimalarials was in 37 patients (40%) rheumatoid arthritis, in 36 patients (39%) systemic lupus erythematosus, in six patients other collagenoses, and in the remaining 14 patients various other rheumatic diseases. A total of 16 patients (17%) received hydroxychloroquine, while the majority received chloroquine (83%). Regular examinations were performed every 6 months. In the analysis three monitoring visits were included: baseline examination, last available examination, and one intermediate examination dividing time into equal intervals. If the medication was discontinued for any reason, the last examination was the examination in which it was decided to stop the drug. A total of 61 patients were seen more than once, 51 patients were followed several times. Mean age when starting follow up was 50.8 (SD 17.6) years, mean follow up time 2.6 (SD 2.9) years. On every examination besides clinical examination, EOG and colour vision testing as described below were performed. Amsler visual field testing was only performed when a maculopathy was suspected.
In total, 51 out of 93 patients were totally normal. A mild retinopathy (as defined above) was found in 38 patients and four patients showed an advanced retinopathy. In patients without retinopathy the mean cumulative dose at last follow up visit was 297 g chloroquine (SD 298 g) and 324 g hydroxychloroquine (228 g). Those exhibiting mild retinopathy had received total doses of 280 g (286 g) chloroquine and 155 g (153 g) hydroxychloroquine, not statistically different from those without retinopathy. Those patients with advanced retinopathy had received a total dose of chloroquine of 250, 365, and 550 g respectively (mean 400 g; p=0.6) and one patient had received 1500 g of hydroxychloroquine. However, only one of these had been previously monitored at our institution. In two of those four cases we had long term records of body weight available and the dose given far exceeded the recommended 3 mg/kg/day for chloroquine and 6.5 mg/kg/day for hydroxychloroquine.
EOG
Electro-oculography was carried out on all patients in accordance with the standards of International Society for Clinical Electrophysiology of Vision (ISCEV).34 Pupil dilatation with 1% tropicamide was performed before pre-adaptation. Horizontal fixation targets were approximately 30° apart, silver-silver chloride electrodes were placed according to ISCEV standards. Recording was performed with a Jaeger-Toennies “multiliner” system (Hoechberg, Germany) and finally the Arden ratio (Arden quotient, AQ) between the lowest dark adapted point (dark trough) and highest light point (light peak) was calculated.34 In all people except one, the EOG was repeated one or more times.
Colour vision test
The computerised colour vision test by Arden was used. The details of the method are described in detail elsewhere.28–30 In brief, the system uses a calibrated 21 inch colour monitor to present random letters as targets to be identified. The letters are of equal luminance to the background, and can only be recognised because their hue differs from the background. The colour of the letters can be varied along a chosen colour-confusion axis. That all stimuli are equiluminant with the background is ensured by a preliminary adjustment (in every patient) of the relative luminance of the red and green and green and blue phosphors. Then a modified binary search is carried out to determine the threshold colour contrast along protan and tritan colour confusion lines. The resulting threshold should not exceed 8.0% in normal probands and is known to be almost independent of age.30 The thresholds of 8.0% for protan and tritan axis were used according to Berninger et al,30 where 7.0% was found as an extreme value for protan axis and 7.9% for tritan axis in normals. The threshold represents a deviation of more than 3 SD for protan and 2 SD for tritan from the normal mean. Completeness of follow up examinations was identical to that of the EOG.
Statistics
Data were collected and analysed using spss 10.0 for Windows (SPSS Inc, Chicago, IL, USA). On all tests p<0.05 was considered significant. Unless otherwise specified for comparisons of means the parametric T test was used. At the begin of analysis a comprehensive graphical analysis of all results by means of scatter plots and box plots was performed to identify possible influencing factors. The patients receiving chloroquine and hydroxychloroquine were combined for assessment of the diagnostic tests, as both drugs show very similar toxicities, although with different probabilities. Receiver operating characteristic (ROC) curves were calculated with spss 10.0 as a statistical standard method to investigate the figure of merit for diagnostic tests. For assessment of diagnostic value the sensitivity and specificity were calculated manually (sensitivity = number of patients where the test result is abnormal and who have the disease/all patients with the disease; specificity = normals with a normal test result/all normals). The positive and negative predictive values were also calculated as common in statistical analysis. For this, it was assumed that the prevalence of retinopathy (that is, occurrence at some time during therapy) in patients receiving chloroquine was 10%.
RESULTS
EOG
The results of EOG testing are summarised in Table 1. The highest sensitivity of 61% is achieved when using an AQ of 180 for discriminating between normal and abnormal EOGs. However, then only 54% specificity is obtained. When a prevalence of 10% retinopathy is assumed, this would yield a positive predictive value of only 13%, which means that only 13% of those patients with an abnormal EOG have retinopathy. The corresponding negative predictive value is 93%—that is, 93% of patients with a normal EOG do not have retinopathy. This has to be compared with the assumed 90% of patients who do not develop retinopathy anyway. Some other criteria like AQ 160% as a limit, or a reduction of AQ more than 20% or 30% over time are listed in Table 1. None of them shows significantly better characteristics for screening. There are no large differences between considering all maculopathies and only advanced maculopathies.
Table 1.
EOG in retinopathy
| EOG criteria | Mild retinopathy | Advanced retinopathy | Normals |
| AQ<180 “abnormal” | 23 | 2 | 23 |
| AQ>180 “normal” | 15 | 2 | 27 |
| Sensitivity 61% | Sensitivity 50% | Specificity 54% | |
| AQ<160 “abnormal” | 12 | 2 | 9 |
| AQ>160 “normal” | 26 | 2 | 41 |
| Sensitivity 32% | Sensitivity 50% | Specificity 82% | |
| Reduction of AQ>20% “abnormal” | 8 | 6 | |
| Reduction of AQ<20% “normal” | 20 | 1 | 26 |
| Sensitivity 29% | * | Specificity 81% | |
| Reduction of AQ>30% “abnormal” | 3 | 5 | |
| Reduction of AQ<30% “normal” | 25 | 1 | 27 |
| Sensitivity 11% | * | Specificity 84% |
Sensitivity and specificity of EOG to screen for chloroquine retinopathy. Different cut-off criteria are listed.
*Cannot be assessed validly due to the low patient number.
Colour vision
Analysis of the Arden colour vision test is summarised in Table 2. While an absolute defect in the protan axis is found in only 22% of cases with mild retinopathy, this criterion yields a sensitivity of 75% in advanced maculopathy. Disturbances of the tritan axis are less specific (67%), but show 100% sensitivity in patients with advanced retinopathy. When looking at acquired colour defects only, they are found to be very specific. As several patients entered the series with disturbances in colour vision and only one patient with advanced retinopathy could be followed while developing retinopathy, the sensitivity cannot be assessed validly in this study. When calculating the positive predictive value, again, for advanced retinopathy an abnormal protan axis yields 41% and an abnormal tritan axis yields 25%. For the negative predictive value 96.9% are obtained for protan and 100% for tritan axis. This means that a normal test result on Arden colour test virtually excludes any more advanced retinopathy by antimalarials.
Table 2.
Arden colour vision test in retinopathy
| Test results | Mild retinopathy | Advanced retinopathy | Normals |
| Protan axis “abnormal” | 8 | 3 | 6 |
| Protan axis “normal” | 29 | 1 | 45 |
| Sensitivity 22% | Sensitivity 75% | Specificity 88% | |
| Tritan axis “abnormal” | 22 | 4 | 17 |
| Tritan axis “normal” | 15 | 0 | 34 |
| Sensitivity 60% | Sensitivity 100% | Specificity 67% | |
| Acquired colour defect | [3] | 1 | 3 |
| No acquired colour defect | [31] | 44 | |
| [Sensitivity 10%]* | * | Specificity 94% |
Sensitivity and specificity of the computerised Arden colour test to screen for chloroquine retinopathy.
*Cannot be assessed validly due to the low patient number.
Corneal deposits
A distinct cornea verticillata was seen in five of 51 patients without maculopathy, in five of 38 patients with mild retinopathy and in two of the four patients with advanced maculopathy. This yields 13% sensitivity for mild macular changes and remarkable 50% sensitivity for advanced retinopathy at 90% specificity. For advanced retinopathy a positive predictive value of 36% and a negative predictive value of 94% is obtained.
Effect of age
To investigate if age related changes have a role in the diagnostic tests, regression analysis was performed. For the EOG no significant effect was found in the patients treated with antimalarials: EOG AQ = 198 − 0.47 × age. For the colour test a significant, but small effect of age was found for both, the protan (p=0.03) and larger for the tritan axis (p<0.001).
Protan threshold = 3.75 + 0.064 × age
Tritan threshold = 1.81 + 0.129 × age
When only the subgroup of patients who never developed any retinopathy was examined, a similarly significant, but smaller effect in size remained:
Protan threshold = 4.56 + 0.037 × age
Tritan threshold = 3.60 + 0.070 × age
ROC analysis
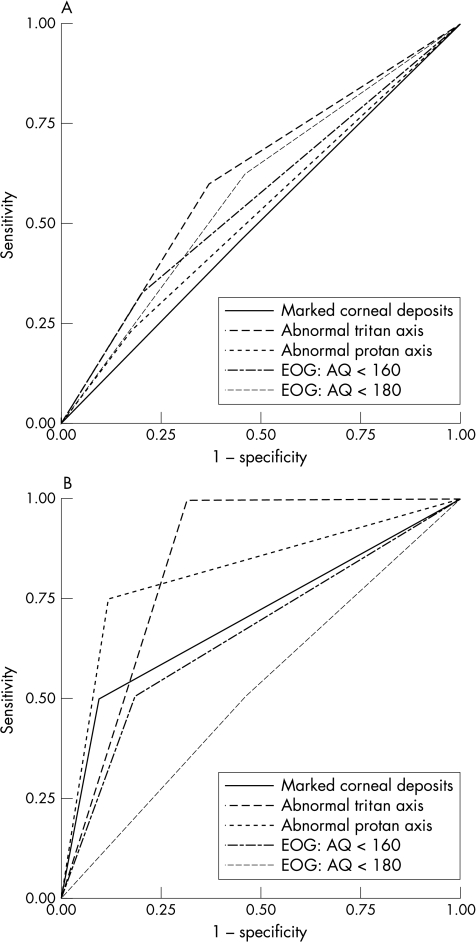
The results obtained with the given cut-off limits are summarised as ROC curves in Figure 1. While for mild retinopathies (Fig 1A) no test shows suitable test characteristics (all curves are close to the diagonal), for advanced retinopathies (Fig 1B) a disturbance of protan and tritan axis in the Arden colour test shows a distinct curve. The area under the curve representing diagnostic quality is highest for tritan changes (0.840) and still very high for changes in protan axis (0.815).
Figure 1.
(A) ROC curves for mild retinopathy. (B) ROC curves for advanced retinopathy. One can see that in mild retinopathy (A) none of the tests shows a receiver operating characteristic (ROC) curve distinctly different from the diagonal and is therefore suitable for screening. In advanced retinopathy (B) as defined by existing visual field defects, a defect colour vision in protan and tritan axis offer good characteristics for screening. Marked corneal deposits show similar fair characteristics as does the best criteria using the EOG, an AQ of smaller than 160%. The other absolute and relative cut offs for the EOG show even worse test characteristics.
Optimising thresholds
To investigate, whether the cut-off points derived from studies on normal people and previous literature results can be further optimised, ROC analysis was performed. The patients on antimalarials without any sign of retinopathy served as controls. For mild retinopathies the best test characteristics are obtained for a change in the tritan axis. A threshold of 6.2% provides 75% sensitivity and 39% specificity, a threshold of 7.0% gives 64% sensitivity and 63% specificity. The test characteristics for all other tests are less suitable as shown above.
For advanced retinopathy both the tritan and protan axis show acceptable test characteristics. The screening characteristics of EOG are no better than chance and therefore will not be shown. For the tritan axis a threshold of 8.5% still gives 100% sensitivity and 75% specificity, a threshold of 14.8% yields 75% sensitivity and 94% specificity. For the protan axis, a threshold of 6.1% yields 100% sensitivity at 62% specificity and a threshold of 10.0% gives 75% sensitivity and 91% specificity.
ANOVA
To account for possible cross correlations an analysis of variance (ANOVA) was calculated. The covariates and factors included were the age, accumulated total dose of antimalarials, AQ of the EOG, protan threshold, tritan threshold, and occurrence of marked corneal deposits. For prediction of a mild retinopathy occurrence of marked corneal deposits was the only significant factor (p=0.04), while all other tests yielded not statistically significant results. For prediction of an advanced retinopathy a disturbance of the protan axis was highly significant (p=0.01), while all other factors were not statistically significant. The high R2 of 0.45 indicates a good overall prediction of the model.
DISCUSSION
Electrophysiology
Testing of patients taking chloroquine with the EOG was introduced early and showed promising first results.18,35 The EOG is believed to show the interaction between retinal pigment epithelium and rods of the human eye36 and was therefore considered to be a valid diagnostic tool.18,37 However, it soon was noticed that screening for antimalarial toxicity by EOG requires a baseline testing owing to high inter-individual variations.18 It also became obvious, that an Arden quotient (AQ) of 180% is not an appropriate cut off for diagnostic testing.21,38 Because of 10% intra-individual variance a cut off of 20% reduction of AQ was proposed.23,39 Furthermore, it was noted that a reduction of EOG occurred frequently not only because of chloroquine medication but also by the course of the rheumatic disease itself.24,25,40,41 Therefore in the present study the “normals” were defined as patients treated for rheumatic diseases but without any evidence of chloroquine retinopathy. But even given this control group, a rather limited diagnostic value of EOG was found. An absolute cut off of AQ=180% was found completely unsuitable and a cut off of AQ=160% yielded good specificity but very low sensitivity. No improvement could be achieved by using relative reductions within the same patient or ROC analysis. Therefore, given the relatively time consuming process of EOG measurement including dark adaptation34 its use as a screening test appears little justified.
The ERG was also evaluated for screening for chloroquine toxicity,18,19 but never found to be a more suitable screening test than the EOG.18,23–25,42,43 It therefore was not evaluated in this study although it is nowadays performed with similar frequency as the EOG.26 Until now only case reports regarding the pattern ERG are available,44 while the multifocal techniques such as the multifocal ERG seem to be promising for detecting chloroquine retinopathy.45,46 However, multifocal techniques are not yet routinely applied and further studies with larger patient series are required.
Colour vision
Defects in colour vision are known to occur with antimalarial treatment that causes retinal toxic changes47 and may precede fundus changes.31 However, reported changes may be subtle and frequently are not detected by conventional colour testing.8,33,35,48–50 The first changes resulting from retinal toxicity occur with blue-yellow (that is, tritan) colours, while protan defects occur in more advanced cases.51,52 This explains why every colour test is not equally applicable. Recently, Vu and Easterbrook17 compared different clinical colour tests on patients with established retinopathy and normal patients with rheumatic diseases. They found that the Standard Pseudoisochromatic Plates Part 2 (SPP-2) had the best test characteristics for screening, yielding a sensitivity of 93% and a specificity of 88%.
In contrast with this plate based test, in our study colour vision testing is performed by a computerised colour test. Besides several other testing possibilities, this test allows a very exact and fast determination of individual colour vision on the protan and tritan axis.28–30 Based on our patient database this method allowed a sensitivity of 100% with 67% specificity for defects on the tritan axis, while defects on the protan axis yielded 75% sensitivity and 88% specificity. With adjustment of the thresholds even 100% sensitivity at 75% specificity can be obtained using the tritan axis. Those results are comparable to those of the SPP-2 published by Vu et al.17 However, not all patients were seen shortly after starting chloroquine medication so unfortunately no valid evaluation of the intra-individual changes during therapy was possible. This fact is important as some, especially male patients, may have an inborn defect in colour vision thus falsely reducing specificity.30 One main advantage of the computerised test used is that even in those patients with a congenital or possible deficiency due to the rheumatic disease itself a further reduction in colour vision can be monitored. The findings of Vu et al17 that earlier defects are more on the tritan axis, while more advanced defects also involve the protan axis, are confirmed by our results. On the other hand tritan changes are less specific, as they are more easily affected by other systemic and ocular changes such as media opacities by, for example, cataracts.54 The computerised colour test used in our study has the advantage that it is relatively insensitive to disturbances in colour perception due to age.30 Still, some age related changes can be found. They lie in the same range as known from normal probands.30,55 In an ANOVA, however, this small effect of age proved insignificant compared to the large changes in developing retinopathy.
Corneal deposits
Corneal deposits are known to occur in the corneal epithelium and superficial stroma. In experiments with albino rats a more peripheral localisation and a dose dependent effect was found.56 The dose dependent corneal changes in corneal epithelium and anterior stroma could be confirmed on patients in vivo by confocal microscopy.7 It is also known that up to 95% of patients on chloroquine (in contrast with approximately 10% of patients taking hydroxychloroquine) exhibit corneal deposits.5 A correlation with overdosage, especially in hydroxychloroquine, has been discussed,5 but unfortunately most reports do not even mention corneal status.10,11,57,58 The importance of carefully examining the cornea for a verticillata4,5,27 as it may hint at retinopathy is supported by our results: for advanced retinopathy 50% sensitivity and 90% specificity were obtained for a marked cornea verticillata, which is comparable to the results of EOG testing. In ANOVA the occurrence of corneal deposits was even found a statistically significant predictor of mild retinopathy. Therefore careful slit lamp examination (with dilated pupil) of the cornea gives easily obtainable, valuable additional information on the probability of retinopathy.
Visual field testing
Visual field testing is frequently performed for monitoring,26 since it was first proposed by Hart et al in 1984.14 Definite maculopathy is defined by Easterbrook as reproducible bilateral field defects,8,59 while early maculopathy does not show visual field defects.60 The use of Amsler grid for testing is inexpensive and fast and therefore is frequently recommended.13,16,26,27,32 However, it is very dependent on the compliance of the patient and non-specific as approximately 6% of the normal population has defects.49 Amsler grid testing may still give valuable hints and allows a less frequent use of automated static perimetry.27 Although routinely many centres use static 10-2 automated perimetry, only few reports exist regarding its suitability for screening. Easterbrook found 91% sensitivity and 58% specificity for a red perimetry,4 which is in the range of colour vision tests. Therefore perimetry often is only performed, if other risk factors for retinopathy such as abnormal Amsler testing, abnormal colour vision, or long lasting therapy are present.27 However, further prospective studies regarding 10-2 white perimetry are needed.
Experimental data
Several experimental studies on animals and cell cultures may influence the clinical attitude towards further screening considerations for retinopathy. While early studies assumed a binding of chloroquine to melanin caused retinal toxicity61,62 it is nowadays well known that melanin is not at all concerned in ocular toxicity.63 This is supported by the fact, that retinopathy can be reproduced equally both in albino and in pigmented rabbits, rats and cats.64–68 The early histopathological reports of retinal changes showed membranous cytoplasmic bodies predominantly in the ganglion cells of the retina in humans,69 monkey70 and rodents,71 whereas the pigment epithelium showed only changes in very advanced retinopathy. Regarding the underlying mechanism, lipid complex accumulation causes ganglion cells to develop complex-containing lysozomes, Mueller and bipolar cells being first affected.65 It appears that changes of the retinal pigment epithelium occur only in later stages as a secondary effect.63 As far as it is known, chloroquine forms complexes with gangliosides impairing further degradation72 finally leading to a accumulation of lipid complexes in the neuroretina.73,74 Ultimately this may cause irreversible damage to rods and cones even after cessation of the drug.75,76 Here, the interference of chloroquine with DNA may play a part.62,73,74
From this mechanism of first impairing ganglion cell function it becomes clear that in early chloroquine retinopathy screening strategies must focus on those cells. This may explain why the normal EOG and ERG are not as effective as expected but colour vision is a sensitive test as it is in glaucoma.29 Of the electrophysiological tests, theoretically the pattern ERG should be the most effective.77 As the retinopathy occurs predominantly in the macula, multifocal techniques are promising45 and visual field testing can be limited to the central 10°.
CONCLUSIONS
When the recommended 3 mg chloroquine and 6.5 mg hydroxychloroquine per kilogram ideal body weight are not exceeded, the risk for retinopathy is low.78 As hydroxychloroquine appears to be very safe, less frequent or even no monitoring is necessary for this drug. Regular screening is, however, indicated for patients taking chloroquine and should consist of vision testing, corneal and fundus examination, and Amsler as well as colour vision testing. Further tests such as fundus photography, automated visual field testing, and fluorescein angiography may be required in suspect cases to differentiate other retinal changes. It could be shown that EOG screening is of limited value, whereas the proposed automated colour vision test offers high diagnostic value. It is best to have a baseline examination when retinopathy can still be excluded because of a low total chloroquine dose—for example, within the first 6 months.
Acknowledgments
The authors thank Christel Hörmann for expert technical assistance and the reviewers for very helpful comments. Parts of this study contributed to the doctoral thesis of Kambiz Samari-Kermani at the medical faculty of the Ludwig-Maximilians-Universität, Munich.
Presented in part at the European Association for Vision and Eye Research annual meeting, Alicante, Spain, 2002.
The authors do not have any commercial interest in any of the materials and methods used in this study.
REFERENCES
- 1.Cambioggi A. Unusual ocular lesions in a case of systemic lupus erythematosis. Arch Ophthalmol 1957;57:451–3. [DOI] [PubMed] [Google Scholar]
- 2.Hobbs H, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet 1959;2:478. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein HN. Ophthalmologic considerations and testing in patients receiving long-term antimalarial therapy. Am J Med 1983;75:25–34. [DOI] [PubMed] [Google Scholar]
- 4.Easterbrook M. Detection and prevention of maculopathy associated with antimalarial agents. Int Ophthalmol Clin 1999;39:49–57. [DOI] [PubMed] [Google Scholar]
- 5.Easterbrook M. Is corneal deposition of antimalarial any indication of retinal toxicity? Can J Ophthalmol 1990;25:249–51. [PubMed] [Google Scholar]
- 6.Francois J, Maudgal MC. Experimentally induced chloroquine retinopathy in rabbits. Am J Ophthalmol 1967;64:886–93. [DOI] [PubMed] [Google Scholar]
- 7.Slowik C, Somodi S, von Gruben C, et al. [Detection of morphological corneal changes caused by chloroquine therapy using confocal in vivo microscopy.] Ophthalmologe 1997;94:147–51. [DOI] [PubMed] [Google Scholar]
- 8.Easterbrook M. Ocular effects and safety of antimalarial agents. Am J Med 1988;85:23–9. [DOI] [PubMed] [Google Scholar]
- 9.Fielder A, Graham E, Jones S, et al. Royal College of Ophthalmologists guidelines:ocular toxicity and hydroxychloroquine. Eye 1998;12(Pt 6):907–9. [DOI] [PubMed] [Google Scholar]
- 10.Brinkley Jr JR, Dubois EL, Ryan SJ. Long-term course of chloroquine retinopathy after cessation of medication. Am J Ophthalmol 1979;88:1–11. [DOI] [PubMed] [Google Scholar]
- 11.Mavrikakis M, Papazoglou S, Sfikakis PP, et al. Retinal toxicity in long term hydroxychloroquine treatment. Ann Rheum Dis 1996;55:187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruess AF, Schachat AP, Nicholl J, et al. Chloroquine retinopathy. Is fluorescein angiography necessary? Ophthalmology 1985;92:1127–9. [PubMed] [Google Scholar]
- 13.Easterbrook M. The use of Amsler grids in early chloroquine retinopathy. Ophthalmology 1984;91:1368–72. [DOI] [PubMed] [Google Scholar]
- 14.Hart Jr WM, Burde RM, Johnston GP, et al. Static perimetry in chloroquine retinopathy. Perifoveal patterns of visual field depression. Arch Ophthalmol 1984;102:377–80. [DOI] [PubMed] [Google Scholar]
- 15.Easterbrook M, Trope G. Value of Humphrey perimetry in the detection of early chloroquine retinopathy. Lens Eye Toxic Res 1989;6:255–68. [PubMed] [Google Scholar]
- 16.Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy:a report by the American Academy of Ophthalmology. Ophthalmology 2002;109:1377–82. [DOI] [PubMed] [Google Scholar]
- 17.Vu BL, Easterbrook M, Hovis JK. Detection of color vision defects in chloroquine retinopathy. Ophthalmology 1999;106:1799–803; discussion 804. [DOI] [PubMed] [Google Scholar]
- 18.Kolb H. Electro-oculogram findings in patients treated with antimalarial drugs. Br J Ophthalmol 1965;49:573–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sverak J, Erbenova Z, Peregrin J, et al. [The ERG and EOG potentials aft a long-term Resochin therapy.] Klin Monatsbl Augenheilkd 1970;157:389–92. [PubMed] [Google Scholar]
- 20.Graniewski-Wijnands HS, van Lith GH, Vijfvinkel-Bruinenga S. Ophthalmological examination of patients taking chloroquine. Doc Ophthalmol 1980;48:231–4. [DOI] [PubMed] [Google Scholar]
- 21.Percival SP. The ocular toxicity of chloroquine. Trans Ophthalmol Soc UK 1967;87:355–7. [PubMed] [Google Scholar]
- 22.Reijmer CN, Tijssen JG, Kok GA, et al. Interpretation of the electro-oculogram of patients taking chloroquine. Doc Ophthalmol 1980;48:273–6. [DOI] [PubMed] [Google Scholar]
- 23.Van Lith GH. Electro-ophthalmology and side-effects of drugs. Doc Ophthalmol 1977;44:19–21. [DOI] [PubMed] [Google Scholar]
- 24.Pinckers A, Broekhuyse RM. The EOG in rheumatoid arthritis. Acta Ophthalmol (Copenh) 1983;61:831–7. [DOI] [PubMed] [Google Scholar]
- 25.Gouras P, Gunkel R. The EOG in chloroquine and other retinopathies. Arch Ophtalmol 1963;70:91–100. [DOI] [PubMed] [Google Scholar]
- 26.Mazzuca SA, Yung R, Brandt KD, et al. Current practices for monitoring ocular toxicity related to hydroxychloroquine (Plaquenil) therapy. J Rheumatol 1994;21:59–63. [PubMed] [Google Scholar]
- 27.Easterbrook M. Current concepts in monitoring patients on antimalarials. Aust NZ J Ophthalmol 1998;26:101–3. [DOI] [PubMed] [Google Scholar]
- 28.Arden G, Gunduz K, Perry S. Color vision testing with a computer graphics system:preliminary results. Doc Ophthalmol 1988;69:167–74. [DOI] [PubMed] [Google Scholar]
- 29.Gunduz K, Arden GB, Perry S, et al. Color vision defects in ocular hypertension and glaucoma. Quantification with a computer-driven color television system. Arch Ophthalmol 1988;106:929–35. [DOI] [PubMed] [Google Scholar]
- 30.Berninger T, Drobner B, Hogg C, et al. [Color vision in relation to age:a study of normal values.] Klin Monatsbl Augenheilkd 1999;215:37–42. [DOI] [PubMed] [Google Scholar]
- 31.Nozik RA, Weinstock FJ, Vignos PJ. Ocular complications of chloroquine. A series and case presentation with a simple method for early detection of retinopathy. Am J Ophthalmol 1964;58:774–8. [PubMed] [Google Scholar]
- 32.Niemeyer G, Fruh B. [Examination strategies in the diagnosis of drug-induced retinal damage.] Klin Monatsbl Augenheilkd 1989;194:355–8. [DOI] [PubMed] [Google Scholar]
- 33.Carr RE, Gouras P, Gunkel RD. Chloroquine retinopathy. Early detection by retinal threshold test. Arch Ophthalmol 1966;75:171–8. [DOI] [PubMed] [Google Scholar]
- 34.Marmor MF, Zrenner E. Standard for clinical electro-oculography. International Society for Clinical Electrophysiology of Vision. Doc Ophthalmol 1993;85:115–24. [DOI] [PubMed] [Google Scholar]
- 35.Henkind P, Carr RE, Siegel IM. Early chloroquine retinopathy:clinical and functional findings. Arch Ophtalmol 1964;71:157–65. [DOI] [PubMed] [Google Scholar]
- 36.Arden GB, Kelsey JH. J Physiol 1962;161:189; 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arden GB, Friedmann AI, Kolb H. Anticipation of chloroquine retinopathy. Lancet 1962;1:1164–65. [DOI] [PubMed] [Google Scholar]
- 38.Graniewski-Wijnands HS, van Lith GH, Vijfvinkel-Bruinenga S. Ophthalmological examination of patients taking chloroquine. Doc Ophthalmol 1979;48:231–4. [DOI] [PubMed] [Google Scholar]
- 39.Van Lith GH, Mak GT, Wijnands H. Clinical importance of the electro-oculogram with special reference to the chloroquine retinopathy. Bibl Ophthalmol 1976:2–9. [PubMed]
- 40.Reijmer CN, Tijssen JG, Kok GA, et al. Interpretation of the electro-oculogram of patients taking chloroquine. Doc Ophthalmol 1979;48:273–6. [DOI] [PubMed] [Google Scholar]
- 41.Bishara SA, Matamoros N. Evaluation of several tests in screening for chloroquine maculopathy. Eye 1989;3(Pt 6):777–82. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein HN. Chloroquine ocular toxicity. Surv Ophthalmol 1967;12:415–47. [PubMed] [Google Scholar]
- 43.Infante R, Martin DA, Heckenlively JR. Hydroxychloroquine and retinal toxicity. Doc Ophthalmol Proc Ser 1983;37:121–6. [Google Scholar]
- 44.Cursiefen C, Grunert U, Junemann A. [Chloroquine-induced bull’s eye maculopathy without electrophysiologic changes.] Klin Monatsbl Augenheilkd 1997;210:400–1. [DOI] [PubMed] [Google Scholar]
- 45.Kellner U, Kraus H, Foerster MH. Multifocal ERG in chloroquine retinopathy:regional variance of retinal dysfunction. Graefes Arch Clin Exp Ophthalmol 2000;238:94–7. [DOI] [PubMed] [Google Scholar]
- 46.Wolfelschneider P, Kohen L, Wiedemann P. [Maculopathy in long-term chloroquine therapy.] Ophthalmologe 1998;95:186–7. [DOI] [PubMed] [Google Scholar]
- 47.Okun E, Gouras P, Bernstein HN, et al. Chloroquine retinopathy:a report of eight cases with ERG and dark-adaptation findings. Arch Ophtalmol 1963;58:774–8. [DOI] [PubMed] [Google Scholar]
- 48.Nylander U. Ocular damage in chloroquine therapy. Acta Ophthalmol (Copenh) 1966;44:335–48. [PubMed] [Google Scholar]
- 49.Percival SP, Meanock I. Chloroquine:ophthalmological safety, and clinical assessment in rheumatoid arthritis. Br Med J 1968;3:579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartel PR, Roux P, Robinson E, et al. Visual function and long-term chloroquine treatment. S Afr Med J 1994;84:32–4. [PubMed] [Google Scholar]
- 51.Jaeger W. [Acquired colour-vision-deficiencies caused by side-effects of pharmacotherapy (author’s transl).] Klin Monatsbl Augenheilkd 1977;170:453–60. [PubMed] [Google Scholar]
- 52.Grutzner P. Acquired color vision defects secondary to retinal drug toxicity. Ophthalmologica 1969;158(Suppl):592–604. [PubMed] [Google Scholar]
- 53.Feichtner JJ, Berry AJ, Simkin PA. Vision and taste deficits in rheumatoid arthritis. Arthritis Rheum 1980;23:672. [Google Scholar]
- 54.Fristrom B, Lundh BL. Colour contrast sensitivity in cataract and pseudophakia. Acta Ophthalmol Scand 2000;78:506–11. [DOI] [PubMed] [Google Scholar]
- 55.Arden GB, Hall MJ. Does occupational exposure to argon laser radiation decrease colour contrast sensitivity in UK ophthalmologists? Eye 1995;9(Pt 6):686–96. [DOI] [PubMed] [Google Scholar]
- 56.Francois J, Maudgal MC. Experimental chloroquine keratopathy. Am J Ophthalmol 1965;60:459–64. [DOI] [PubMed] [Google Scholar]
- 57.Tobin DR, Krohel G, Rynes RI. Hydroxychloroquine. Seven-year experience. Arch Ophthalmol 1982;100:81–3. [DOI] [PubMed] [Google Scholar]
- 58.Mills PV, Beck M, Power BJ. Assessment of the retinal toxicity of hydroxychloroquine. Trans Ophthalmol Soc UK 1981;101:109–13. [PubMed] [Google Scholar]
- 59.Easterbrook M. The ocular safety of hydroxychloroquine. Sem Arthritis Rheum 1993;23:62–7. [DOI] [PubMed] [Google Scholar]
- 60.Percival SP, Behrman J. Ophthalmological safety of chloroquine. Br J Ophthalmol 1969;53:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potts AM. Further studies concerning the accumulation of polycyclic compounds on uveal melanin. Invest Ophthalmol 1964;3:399. [PubMed] [Google Scholar]
- 62.Gonasun LM, Potts AM. In vitro inhibition of protein synthesis in the retinal pigment epithelium by chloroquine. Invest Ophthalmol 1974;13:107–15. [PubMed] [Google Scholar]
- 63.Leblanc B, Jezequel S, Davies T, et al. Binding of drugs to eye melanin is not predictive of ocular toxicity. Regul Toxicol Pharmacol 1998;28:124–32. [DOI] [PubMed] [Google Scholar]
- 64.Francois J, Maudgal MC. Experimental chloroquine retinopathy. Ophthalmologica 1964;148:442–52. [DOI] [PubMed] [Google Scholar]
- 65.Gregory MH, Rutty DA, Wood RD. Differences in the retinotoxic action of chloroquine and phenothiazine derivatives. J Pathol 1970;102:139–50. [DOI] [PubMed] [Google Scholar]
- 66.Legros J, Rosner I. [Electroretinographic modifications in albino rats after chronic administration of toxic doses of hydroxychloroquine and desethylhydroxychloroquine.] Arch Ophtalmol Rev Gen Ophtalmol 1971;31:165–80. [PubMed] [Google Scholar]
- 67.Kuhn H, Keller P, Kovacs E, et al. Lack of correlation between melanin affinity and retinopathy in mice and cats treated with chloroquine or flunitrazepam. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1981;216:177–90. [DOI] [PubMed] [Google Scholar]
- 68.Ivanina TA, Zueva MV, Lebedeva MN, et al. Ultrastructural alterations in rat and cat retina and pigment epithelium induced by chloroquine. Graefes Arch Clin Exp Ophthalmol 1983;220:32–8. [DOI] [PubMed] [Google Scholar]
- 69.Ramsey MS, Fine BS. Chloroquine toxicity in the human eye. Histopathologic observations by electron microscopy. Am J Ophthalmol 1972;73:229–35. [DOI] [PubMed] [Google Scholar]
- 70.Rosenthal AR, Kolb H, Bergsma D, et al. Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci 1978;17:1158–75. [PubMed] [Google Scholar]
- 71.Hodgkinson BJ, Kolb H. A preliminary study of the effect of chloroquine on the rat retina. Arch Ophthalmol 1970;84:509–15. [DOI] [PubMed] [Google Scholar]
- 72.Lüllmann-Rauch R, ed. Lipoidosis of the retina due to cationic amphiphilic drugs, rat. Berlin: Springer-Verlag, 1991.
- 73.Meier-Ruge W, Cerletti A. Zur experimentellen Pathologie der Phenothiazin-Retinopathie. Ophthalmologica 1966;151:512–33. [DOI] [PubMed] [Google Scholar]
- 74.Tanenbaum L, Tuffanelli DL. Antimalarial agents. Chloroquine, hydroxychloroquine, and quinacrine. Arch Dermatol 1980;116:587–91. [DOI] [PubMed] [Google Scholar]
- 75.Duncker G, Bredehorn T. Chloroquine-induced lipidosis in the rat retina:functional and morphological changes after withdrawal of the drug. Graefes Arch Clin Exp Ophthalmol 1996;234:378–81. [DOI] [PubMed] [Google Scholar]
- 76.Duncker G, Schmiederer M, Bredehorn T. Chloroquine-induced lipidosis in the rat retina:a functional and morphological study. Ophthalmologica 1995;209:79–83. [DOI] [PubMed] [Google Scholar]
- 77.Arden GB. Comparison of new psychophysics and perimetry with electrophysiological techniques in the diagnosis of glaucoma. Curr Opin Ophthalmol 1993;4:14–21. [PubMed] [Google Scholar]
- 78.Mackenzie AH. Dose refinements in long-term therapy of rheumatoid arthritis with antimalarials. Am J Med 1983;75:40–5. [DOI] [PubMed] [Google Scholar]