Abstract
Aims: To report a case of an unusual retinal vascular morphology in connection with a novel AIPL1 mutation in a patient with Leber’s congenital amaurosis (LCA).
Methods: A patient with LCA and no light perception from birth had both eyes enucleated at the age of 22 years because of excruciating pain. Mutation analysis was performed on known LCA genes. The eyes were processed for casts of the vascular tree, routine histopathology, and electron microscopy.
Results: A novel H82Y (244C→T) mutation and a H90D (286G→C) polymorphism were detected in exon 2 of the AIPL1 gene. Both the cast and the histopathological examination showed dilated retinal vessels, mainly venules, primarily localised in the posterior pole. In the mid-peripheral retina the density of capillaries on the arteriolar side of the microcirculatory units was significantly decreased. The vascular system was seen to gradually attenuate towards the retinal periphery, and to stop at a zone located approximately 4 mm from the ora serrata along the whole circumference. In this zone pigmented aggregates characteristic of retinitis pigmentosa were seen to ensheath the retinal vessels. The photoreceptors were almost totally absent and retinal gliosis was present. A decreased number of ganglion cells and an increased vacuolisation of the nerve fibre layer were observed. The retinal pigment cells and Bruch’s membrane appeared normal in all regions.
Conclusion: An unusual retinal vascular morphology in an LCA patient is presented and possible pathogenic mechanisms of the findings are discussed.
Keywords: Leber’s congenital amaurosis, histology, vessels, retina
L eber’s congenital amaurosis (LCA) comprises a group of hereditary disorders characterised by low vision or blindness from birth or early infancy. The group is clinically heterogeneous and the fundus in particular is highly variable.1 Genetic heterogeneity has been inferred, based on the observation of a couple who both suffered from LCA and had unaffected children,2 and recently genetic heterogeneity has been confirmed at the molecular level. Currently, seven disease causing genes have been reported: RetGC1,3 RPE65,4 CRX,5 AIPL1,6 LRAT,7 CRB1,8 RPGRIP,9 and two further loci: LCA3 on 14q2410 and LCA5 on 6q11-16.11 Clinical indications of the gene involved occur with mutations in RetGC1 causing congenital blindness,12 in RPE65 causing early onset of severe rod and cone degeneration,13 and in AIPL1 causing keratoconus and early onset of severe retinal degenerations.6 Histological reports are relatively sparse. The photoreceptors seem to be primarily affected,14 in contrast with the cellular localisation of the currently reported gene products. Later in the course of the disease the retina and the choroid become atrophic and the vessels sclerotic.15,16
In the present case an unusual retinal vascular morphology is presented and possible pathogenic mechanisms are discussed.
MATERIALS AND METHODS
Clinical history
The patient was son of unrelated parents and his 4 year older sister had LCA diagnosed at the age of 1 year. At the age of 5 months he therefore underwent a clinical examination which showed no light perception in both eyes. Ophthalmoscopy revealed greyish atrophic retinas but normally calibrated retinal vessels. Electroretinography showed extinguished signals in both eyes.
At the age of 22 years the patient was admitted because of constant headache and pain in both eyes. The patient tried to relieve the pain by poking the eyes. The clinical examination disclosed bilateral nystagmus and no light perception. Slit lamp examination showed bilateral keratoconus and posterior polar cataract. Ophthalmoscopy gave a slightly blurred view of the fundi which had an increased density of retinal vessels in the central and the mid-peripheral retinal areas. However, the far periphery was avascular and with hyperpigmentations dispersed in the retina. The patient had both eyes enucleated, which led to an immediate relief of the pain.
The patient’s older sister was investigated afterwards, and apart from narrow retinal vessels, the clinical examination was normal.
Tissue processing
Immediately after enucleation both eyes were placed with the optic nerve directed upwards, and the central retinal artery was cannulated as previously described.17 After perfusion with heparinised saline to empty the retinal vascular system of blood elements and with 4% formaldehyde for fixation, the retinal vascular system was cast with silicone.18,19
The two eyes were each divided by a section along the horizontal meridian, and the cast vascular tree was documented photographically. One half from one eye was frozen immediately at −80°C.
One half from each eye was dehydrated in ethanol and embedded in paraffin. Sections were cut at 3 μm and mounted on glass slides. The sections were stained with haematoxylin and eosin (HE). The fourth half eye was fixated in glutaraldehyde. A 2 × 2 mm piece of the retina and the adjacent retinal pigment epithelium were excised along the sectioning line at the horizontal meridian from three regions—that is, the macular area, the mid-peripheral area, and the avascular periphery. These three specimens were post-fixed in OsO4 and embedded in Epon.
Molecular genetics
Blood samples were taken from the patient, his sister, and their parents after informed consent had been obtained according to the Declaration of Helsinki.
DNA was extracted from peripheral blood lymphocytes according to a previously reported method.20 DNA samples were subjected to polymerase chain reaction (PCR) using oligonucleotide primers for RetGC1, RPE65, NUB1, and AIPL1 designed by one of the authors (MP) and according to Perrault et al3 and Marlhens et al.21 Subsequently, PCR products were analysed using single strand conformation polymorphism analysis (SSCP) as previously described.22 PCR products presenting aberrant banding patterns in SSCP were sequenced directly by cycle sequencing using the fluorescent chain termination technique. To confirm the D90H (286G→C) mutation, PCR products of the patient, his relatives, and 48 controls were tested for the loss of a NlaIV restriction site.
RESULTS
Molecular genetic findings
SSCP screening excluded sequence variations in RPE65 and RetGC1 in the patient. A novel H82Y (244C→T) mutation and a D90H (286G→C) polymorphism were detected in exon 2 of the AIPL1 gene. Both mutations were present in the patient and his affected sister. The D90H polymorphism was inherited from the maternal side while the H82Y mutation was of paternal origin. No further mutation could be detected.
Vascular morphology
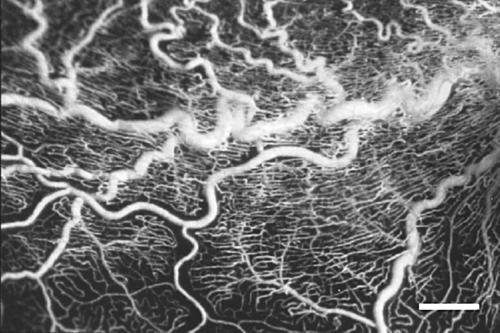
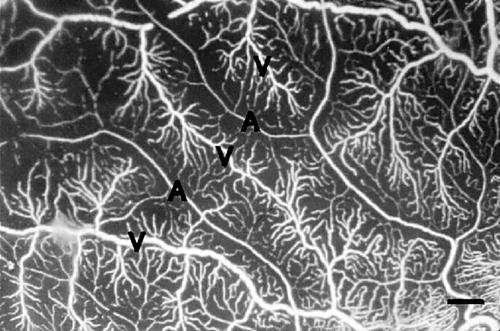
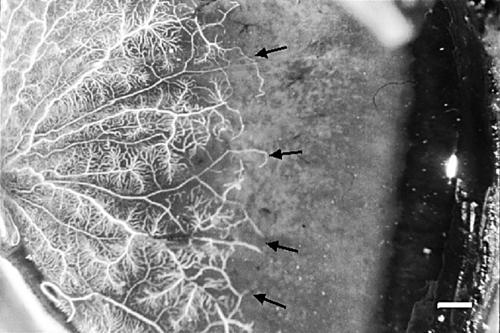
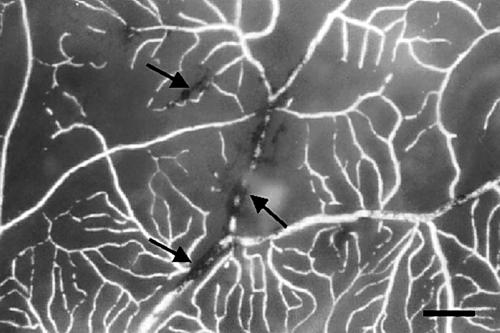
The casts of the retinal vascular system showed the same pattern in the two eyes. Generally, the larger vessels, and especially the venules, displayed increased tortuosity (Fig 1) compared to vessels in normal retinas, but tumour-like aggregates of vessels were not observed. In the macular area there was a pathologically increased vascular density of the capillaries on the venous side of microcirculatory units and of the post-capillary venules. In the mid-peripheral retina the density of capillaries on the venous side of microcirculatory units was normal, but was decreased on their arteriolar counterparts (Fig 2) compared to normal retinas. The vascular system was seen to further gradually attenuate towards the retinal periphery. A totally avascular zone was located approximately 4 mm from the ora serrata along the whole circumference (Fig 3). In this zone hyperpigmentations characteristic of retinitis pigmentosa were seen to ensheath the former retinal vessels (Fig 4).
Figure 1.
Part of the lower temporal arcade (large horizontal vessels in the middle of the image). The venules display pronounced tortuosity. The capillary density is high, including the radial peripapillary network (bar = 500 μm).
Figure 2.
Higher magnification of the vessels radiating towards the periphery. The vascular density increases from the arteriolar (A) to the venular (V) side of the microcirculatory units (bar = 50 μm).
Figure 3.
Nasal retina of the right eye. The vessels terminate (arrows) at an avascular zone approximately 4 mm wide at the ora serrata (bar = 1 mm).
Figure 4.
Vessels near the peripheral avascular zone are ensheathed by lines of pigment (arrows) (bar = 500 μm).
Light microscopy
Anterior segment
The cornea disclosed a partly irregular epithelium with facets filling focal disruptions in Bowman’s layer and a minor stromal thinning. The corneal findings were consistent with keratoconus. The ciliary muscle was atrophic. The lens was slightly cataractous in the equatorial and posterior region.
The remainder of the anterior segment showed no pathological changes.
Posterior segment
The choriocapillaris was normal. The arteries of the choroidal stroma displayed hyaline thickening and the venules in the posterior and equatorial parts were dilated.
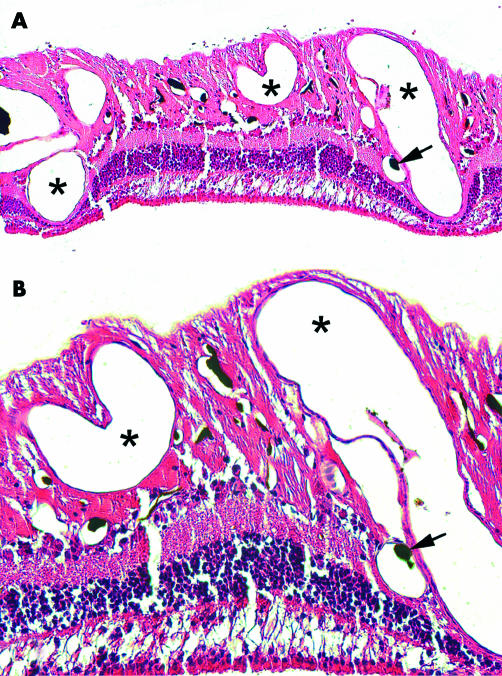
The retina showed the most marked changes. Arterioles, capillaries and, especially, venules were increased both in number and diameter especially in the posterior region (Fig 5). In the most dense areas the vascular meshwork resembled an angiomatous malformation. Most vessels were located in the inner retinal layers, but venules and capillaries were found throughout all retinal layers until the innermost aspects of the outer nuclear layer (Fig 5). The vascular changes were less marked in the mid-periphery. In the anterior portion of the retina the vessels were sclerotic and surrounded by pigmented cells.
Figure 5.
(A) Histological section of retina with abnormal, dense vascular meshwork. Survey. Asterisks on markedly dilated vessels. Arrow on black cast material in a minor arteriole (HE, ×100). (B) Higher magnification demonstrating an area also rich in minor vessels. Marking as in (A) (HE, ×200).
Photoreceptors were almost totally missing and retinal gliosis dominated. The severity of the retinal atrophy was most pronounced in the anterior parts. A decrease in the number of ganglion cells and vacuolisation of the nerve fibre layer were seen.
Clusters of retinal pigment epithelium cells arranged in tubules or at random were localised in the neuroretina. In the periphery the pigmentation had the characteristics of retinitis pigmentosa—that is, clusters of pigments and pigmented cells accumulated around sclerotic vessels. Melanophages were not identified.
The retinal pigment epithelium on Bruch’s membrane appeared normal in all regions and the optic nerve was normal.
Electron microscopy
The study was limited to the retinal vessels and the outer retinal layers.
The retinal vessels in the posterior region and mid-periphery were increased in number and dilated compared to findings in normal eyes; however, all with normal architecture and ultrastructure. Only remnants of outer segments of the photoreceptors were noticed. The retinal pigment epithelial cells appeared morphologically normal in all three areas investigated.
DISCUSSION
Leber’s congenital amaurosis accounts for a least 5% of all inherited retinal diseases.23 It is the most severe form of inherited retinal dystrophy responsible for congenital blindness with an early age of onset.24 LCA is not a homogeneous, well defined entity, but seems to consist of a number of diseases genetically and phenotypically different.1 Seven disease causing genes have been identified so far.3–9 An overview of the currently known mutations is given at www.retina-international.org/sci-news/mutation.htm
The ophthalmoscopic features of LCA are variable and age dependent. A review of 43 cases includes a range of morphologies from normal to retinitis pigmentosa-like pictures, salt and pepper-like changes, chorioretinal atrophy, and macular colobomas as the most common fundus findings.25 The retinal vessel morphology in LCA varies also with age. Normal to attenuated vessels are found in younger individuals.1 However, in a review of 45 patients all adults showed arteriolar narrowing.26
The histological description of LCA is mostly based on single cases or on series of a few patients. The histopathological changes affect predominantly the photoreceptor layer, the ganglion cell layer or the retina diffusively.14 Late in the course of the disease, full thickness retinal and choroidal atrophy are present and the morphology is similar to that seen in retinitis pigmentosa.15
The retinal vessels show hyalinisation,15,16 which was only observed in the anterior, atrophic retinal areas of our case. Focal hyalinisation of the choriocapillaris has also been observed,16 but was absent in the present case.
Few families with a verified AIPL1 mutation have been investigated and have shown a phenotypic variability spanning from normal to cases with keratoconus, retinal atrophy, retinitis pigmentosa, and narrow blood vessels.6,27 The AIPL1 gene product has recently been localised in human rod photoreceptors.28 AIPL1 mutations are estimated to cause approximately 7% of LCA worldwide with the D90H polymorphism being the most frequent in all investigated populations.6 AIPL1 is thought to function as a chaperone because of its homology to FK506 binding proteins.6 The H82Y mutation has not been reported before and its localisation in the N-terminal part of the gene does not allow any evaluation since no functional domains could be identified in this part of the protein.
The involvement of several genetic components, possibly in combination with mutations in the AIPL1 gene, seems to be likely. The case presented is one of five patients from three families presenting either homozygous or compound heterozygous states for D90H and a second AIPL1 mutation in a screen of 60 LCA/early onset retinal degeneration families (unpublished result). The D90H polymorphism has been identified in the single heterozygous state in eight of 48 and in the homozygous state in two of 48 unrelated healthy controls in our mutation screening.
The nature and pathogenesis of LCA has been discussed in the literature and two hypotheses have been proposed—either a primary retinal malformation or a perinatal and postnatal retinal degeneration.14
A morphology of the retinal vessels dominated by tortuosity and an increased number of vessels seen in our patient has to our knowledge never been reported before.
In order to explain these changes in the retinal vessels three hypotheses may be considered: either a mechanical cause—the result of a restricted outflow of blood from the eye/orbit; a tumour-like lesion of the vessels, or a metabolic/genetic disturbance of the retinal tissue leading to reactive or secondary changes in the vessels.
A mechanical compression of the veins from either the eye or the orbit would dilate the retinal venules. However, the vessels in the rest of the eye were without congestion making this explanation unlikely. Neither did the optic nerve disclose an AV shunt as a possible explanation.
A neoplasia or tumour-like reaction of the retinal vessels may be an explanation or the lesion might also be classified as a hamartomatous, or angiomatous, lesion. So called “vasoproliferative tumours of the ocular fundus” do occur as secondary lesions to retinitis pigmentosa.29 However, an elevated retinal mass is seen in these lesions. In the ophthalmoscopic condition “tortuosity of the veins”30 the veins are dilated and tortuous. In the present case the retinal vessels were increased in number as well.
A metabolic/genetic disturbance with an unknown relation to LCA causing a malformation of the retinal vessels may be a hypothetical possibility. The dilated vessels could be caused by a disturbed adaptation and downregulation of vessels normally occurring in retinal degenerations, as a result of a presumed decreased metabolic demand as found in other ocular conditions.19 However, some adaptation has occurred in our patient since the density of capillaries on the arteriolar side of the microcirculatory units was significantly decreased in the mid-periphery, but an avascular zone was present in the periphery. These findings conform to the severity of the retinal atrophy being most pronounced in the anterior segment.
Immature fetal retinal vessels are numerous and the remodelling to form sparser mature vascular trees is not completed at birth. This retinal vessel retraction does occur in regions with higher tissue oxygenation which is present along the arteries.31 A possible disturbed vessel retraction as a result of a regional low retinal metabolism and oxygen demand might explain the retinal vascular morphology observed in this patient.
The periphery of the retina appears in the present case as expected in Leber’s congenital amaurosis. We are not able to explain the presented vascular morphology. Likewise, a possible link between the ocular pain that led to enucleation and the vascular morphology remains obscure. However, the pain may in part be explained by previous episodes of corneal ulcers indicated by the observed corneal epithelial facets.
In conclusion, we present a new retinal vascular morphology of uncertain pathogenicity in a patient with LCA and a novel mutation of AIPL1 of uncertain significance.
Acknowledgments
This study has been supported by a grant from the Danish Support Foundation for the Blind, the medical faculty of the University of Regensburg (ReForM C), the Deutsche Forschungsgemeinschaft (DFG), the Velux Foundation, and the Danish Medical Research Council.
REFERENCES
- 1.Smith D, Oestreicher J, Musarella MA. Clinical spectrum of Leber’s congenital amaurosis in the second to fourth decades of life. Ophthalmology 1990;97:1156–61. [DOI] [PubMed] [Google Scholar]
- 2.Waardenburg PJ, Schappert-Kimmijser J. On various recessive biotypes of Leber’s congenital amaurosis. Acta Ophthalmol (Copenh) 1963;41:317–20. [DOI] [PubMed] [Google Scholar]
- 3.Perrault I, Rozet JM., Calvas P, et al. Retinal-specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nat Genet 1996;14:461–4. [DOI] [PubMed] [Google Scholar]
- 4.Gu S-M, Thompson DA, Srikumari CRS, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet 1997;17:194–7. [DOI] [PubMed] [Google Scholar]
- 5.Freund CL, Wang QL, Chen S, et al. De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. Nat Genet 1998;18:311–2. [DOI] [PubMed] [Google Scholar]
- 6.Sohocki MM, Bowne SJ, Sullivan LS, et al. Mutations in a new photoreceptor-pineal gene on 17p cause Leber congenital amaurosis. Nat Genet 2000;24:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson DA, Li Y, McHenry CL, et al. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nat Genet 2001;28:123–4. [DOI] [PubMed] [Google Scholar]
- 8.Lotery AJ, Jacobson SG, Fishman GA, et al. Mutations in the CRB1 gene cause Leber congenital amaurosis. Arch Ophthalmol 2001;119:415–20. [DOI] [PubMed] [Google Scholar]
- 9.Gerber S, Perrault I, Hanein S, et al. Complete exon-intron structure of the RPGR- interacting protein (RPGRIP1) gene allows the identification of mutations underlying Leber congenital amaurosis. Eur J Hum Genet 2001;9:561–71. [DOI] [PubMed] [Google Scholar]
- 10.Stockton DW, Lewis RA, Abboud EB, et al. A novel locus for Leber congenital amaurosis on chromosome 14q24. Hum Genet 1998;103:328–33. [DOI] [PubMed] [Google Scholar]
- 11.Dharmaraj S, Li Y, Robitaille JM, Silva E, et al. A novel locus for Leber congenital amaurosis maps to chromosome 6q. Am J Hum Genet 2000;66:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preising MN, Rosenberg T, Kellner U, et al. Mutation screening of RetGC1 and RPE65 in patients with LCA and RP. (Abstract) Invest Ophthalmol Vis Sci 2001;42:S644 [Google Scholar]
- 13.Lorenz B, Gyurus P, Preising M, et al. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. (Abstract) Invest Ophthalmol Vis Sci 2000;41:2735–42. [PubMed] [Google Scholar]
- 14.Sullivan TJ, Heathcote JG, Brazel SM, et al. The ocular pathology in Leber’s congenital amaurosis. Aust N Z J Ophthalmol 1994;22:25–31. [DOI] [PubMed] [Google Scholar]
- 15.Kroll AJ, Kuwabara T. Electron microscopy of a retinal abiotrophy. Arch Ophthalmol 1964;71:683–90. [DOI] [PubMed] [Google Scholar]
- 16.Flanders M, Lapointe ML, Brownstein S, et al. Keratoconus and Leber’s congenital amaurosis: a clinicopathological correlation. Can J Ophthalmol 1984;19:310–4. [PubMed] [Google Scholar]
- 17.Bek T, Jensen PK. Three-dimensional structure of human retinal vessels studied by vascular casting. Acta Ophthalmol 1993;71:506–13. [DOI] [PubMed] [Google Scholar]
- 18.Bek T. Transretinal histopathological changes in capillary-free areas of diabetic retinopathy. Acta Ophthalmol 1994;72:409–15. [DOI] [PubMed] [Google Scholar]
- 19.Bek T, Rosenberg T. Clinical pathology and retinal vascular structure in the Bardet-Biedl syndrome. Br J Ophthalmol 1995;79:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marlhens F, Bareil C, Griffoin JM, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet 1997;17:139–41. [DOI] [PubMed] [Google Scholar]
- 22.Preising M, de Laak JP, Lorenz B. Deletion in the OA1 gene in a family with congenital X linked nystagmus. Br J Ophthalmol 2001;85:1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan J, Bonneau D, Frezal J, et al. Clinical and genetic heterogeneity in retinitis pigmentosa. Hum Genet 1990;85:635–42. [DOI] [PubMed] [Google Scholar]
- 24.Foxman SG, Heckenlively JR, Bateman JB, et al. Classification of congenital and early onset retinitis pigmentosa. Arch Ophthalmol 1985;103:1502–6. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder R, Mets MB, Maumenee IH. Leber’s congenital amaurosis. Arch Ophthalmol 1987;105:356–9. [DOI] [PubMed] [Google Scholar]
- 26.Lambert SR, Kriss A, Taylor D, et al. Follow-up and diagnostic reappraisal of 75 patients with Leber’s congenital amaurosis. Am J Ophthalmol 1989;107:624–31. [DOI] [PubMed] [Google Scholar]
- 27.Hameed A, Khalig S, Ismail M, et al. A novel locus for Leber congenital amaurosis (LCA4) with anterior keratoconus mapping to chromosome 17p13. Invest Ophthalmol Vis Sci 2000;41:629–33. [PubMed] [Google Scholar]
- 28.van der Spuy J, Chapple JP, Clark BJ, et al. The Leber congenital amaurosis gene product AIPL1 is localized exclusively in rod photoreceptors of the adult human retina. Hum Mol Genet 2002;11:823–31. [DOI] [PubMed] [Google Scholar]
- 29.Shields CL, Shields JA, Barrett J, et al. Vasoproliferative tumors of the ocular fundus. Arch Ophthalmol 1995;113:615–23. [DOI] [PubMed] [Google Scholar]
- 30.Ballantyne AJ, Michaelson IC. In: Textbook of the fundus of the eye. 2nd ed. Edinburgh and London: E&S Livingstone, 1970:104–5.
- 31.Hughes S, Yang H, Chan-Ling T. Vasularization of the human fetal retina: Roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci 2000;41:1217–28. [PubMed] [Google Scholar]