Abstract
Aim: To compare the efficacy and safety of levofloxacin 0.5% ophthalmic solution (Quixin) with placebo for treatment of bacterial conjunctivitis.
Methods: In this prospective, randomised, placebo controlled, double masked, multicentre study, 249 patients with bacterial conjunctivitis received either 0.5% levofloxacin (n = 126) or placebo (n = 123) for 5 days, administered every 2 hours on days 1–2, then every 4 hours on days 3–5. Cultures were obtained and signs/symptoms evaluated at baseline, interim, and final visits. The end point was the last evaluable observation. Primary microbial outcomes were based on culture results; clinical outcomes were based on resolution of cardinal signs.
Results: 117 patients (60 levofloxacin, 57 placebo) were evaluated. Microbial eradication rates were significantly greater with levofloxacin at all time points, reaching 90% at end point. In a subgroup analysis, differences in eradication rates at end point were most pronounced in children but were also statistically significant for levofloxacin in adults. Clinical cure rates were significantly greater with levofloxacin at final visit and end point. Statistically significant differences favouring levofloxacin were measured at end point for resolution of conjunctival discharge, bulbar conjunctival injection, palpebral conjunctival injection, burning/stinging, itching, and photophobia. Adverse events were similar between groups. Safety composite scores analysed by age indicated significantly fewer children on levofloxacin experienced worsening symptoms.
Conclusions: Levofloxacin 0.5% ophthalmic solution is safe and effective for treatment of bacterial conjunctivitis.
Keywords: bacterial conjunctivitis; levofloxacin; fluoroquinolone, Quixin
Bacterial conjunctivitis can be caused by a number of Gram positive and Gram negative organisms and is characterised by an overgrowth of bacteria on the conjunctival surface with resultant mucosal inflammation.1 Although acute bacterial conjunctivitis is generally considered self limiting, topical antibiotics typically are prescribed based on the assumption that they shorten the duration of the infection, reduce the risk of developing complications, and possibly reduce the risk of epidemic spread of the pathogen.2 Randomised, controlled clinical trials of antibiotics for the treatment of bacterial conjunctivitis are important because they address the question of whether a specific antibiotic therapy actually provides significant clinical benefit to patients.
As treatment of bacterial conjunctivitis is often empirical and initiated before speciation of the ocular pathogen (that is, bacteriological culture), it is important to prescribe an antibiotic with a broad spectrum of antimicrobial activity. In this regard, topical fluoroquinolones have gained increasing use for the treatment of acute bacterial conjunctivitis. The fluoroquinolone family of antibiotics exhibits bactericidal activity by inhibiting two essential bacterial topoisomerase enzymes, DNA gyrase (topoisomerase II) and topoisomerase IV.3,4
Levofloxacin, a newer generation fluoroquinolone, offers several advantages over older generation fluoroquinolones. Levofloxacin is the pure l-enantiomer of ofloxacin. The bactericidal activity of ofloxacin resides primarily in the l-isomer5; this difference is thought to be related to the higher binding affinity of the l-isomer to the DNA–DNA gyrase complex.6 As a result, MIC90 values for levofloxacin against most bacteria are approximately 50% lower than those of ofloxacin.7–9 Levofloxacin appears to have expanded activity against Gram positive organisms (particularly Streptococcus species) compared with the older generation fluoroquinolones, while retaining excellent activity against Gram negative pathogens.9–11
The placebo controlled phase III trial described here was conducted as part of the clinical development of 0.5% levofloxacin ophthalmic solution (Quixin, Santen Inc, Napa, CA, USA) and formed, in part, the basis of its approval by the US Food and Drug Administration (FDA).12 The objective was to evaluate the efficacy and safety of this agent for the treatment of bacterial conjunctivitis.
PATIENTS AND METHODS
Study design
This was a randomised, double masked, placebo controlled study conducted at 14 sites in the United States. The study protocol and informed consent forms were approved by an appropriate institutional review board (IRB) at each institution, and written informed consent was obtained for all enrolled patients. In conjunction with the contract research organisations Clinicor, Inc (Austin, TX, USA) and MedTrials Incorporated (Dallas, TX, USA), this study was conducted under good clinical practice (GCP) standards and according to FDA requirements and guidelines for phase III pivotal trials.
Male and female subjects who were at least 2 years of age and had a clinical diagnosis of bacterial conjunctivitis, characterised by purulent ocular discharge and redness in at least one eye (minimum scores of 1 for conjunctival discharge and conjunctival and/or palpebral injection as described in Table 1), were randomly assigned to receive topical treatment with 0.5% levofloxacin ophthalmic solution or placebo (vehicle) using a 5 day dosing regimen.
Table 1.
Rating scale for assessment of cardinal signs
| Score | Conjunctival discharge | Bulbar conjunctival injection | Palpebral conjunctival injection |
| 0 = absent/normal | No discharge in the lower cul de sac | Normal conjunctival vascular pattern | Normal upper tarsal papillary response |
| 1 = mild | Small amount of mucopurulent or purulent discharge in the lower cul de sac. No matting of eyelids upon awakening in the morning | Diffuse, mild vascular injection, usually without subconjunctival haemorrhages | Diffuse follicular pattern (small follicles) or discrete fine papillary reaction with mild hyperaemia. An upper tarsal papillary response is present but does not obscure underlying details |
| 2 = moderate | Moderate amount of mucopurulent or purulent discharge in the lower cul de sac. Obvious matting together of eyelids in the morning upon awakening | Diffuse hyperaemia that is obvious from a distance and may have scattered petechiae associated subconjunctival haemorrhages | Diffuse follicular reaction (large follicles) or diffuse confluent papillary response, pronounced hyperaemia but without haemorrhage. The upper tarsal papillary response blurs underlying details |
| 3 = severe | Profuse mucopurulent or purulent discharge in the lower cul de sac and marginal tear strip. Eyelids tightly matted together upon arising in the morning, requiring warm soaks to pry the lids apart | “Beet” red eye that may have subconjunctival haemorrhages present in significant numbers and sizes | Marked inflammatory changes in the subconjunctival tissue with evidence of epithelial necrosis. The upper tarsal papillary response completely obscures underlying details |
Microbial and clinical evaluations
At baseline (day 1), demographic information and a detailed medical history were obtained and the following procedures were performed: bacteriological culture (calcium alginate swab of the lower conjunctiva); assessment of ocular signs (biomicroscopy) and symptoms; test of best corrected visual acuity; and undilated fundus examination. The slit lamp examination was performed in some younger patients; however, in those children for whom a slit lamp examination was not possible, a direct ophthalmoscope was used. Conjunctival cultures were analysed by an independent laboratory (Covance Central Laboratory Services, Inc, Indianapolis, IN, USA) and were considered positive only if the colony forming unit (CFU) count for one or more organisms was greater than or equal to the threshold values defined by Cagle and coworkers.13 The transport system utilised Amies medium with charcoal, and the time between inoculation and plating was approximately 2–4 days.
Study medication was dispensed on day 1, and patients returned to the study site for interim (days 3–5) and final (days 6–10) visits, during which biomicroscopy, symptom assessment, test of best corrected visual acuity, and bacteriological cultures were repeated. Fundus examinations were performed through a dilated pupil at the final visit.
Study medication and dosing regimen
A computer generated randomisation schedule was used so that for every four patients, two were assigned to 0.5% levofloxacin and two to placebo (vehicle: benzalkonium chloride (0.005%), sodium chloride, and purified water; hydrochloric acid and/or sodium hydroxide was used to adjust the pH). Study medications were supplied in identical 5 ml bottles with a masked label denoting the patient and protocol numbers, and storage instructions. Patients (and parents or guardians, if appropriate) were instructed to instil 1–2 drops of study medication into the affected eye(s) every 2 hours (up to eight times per day) while awake on days 1 and 2, then every 4 hours (up to four times per day) while awake on days 3–5.
Efficacy assessments
Treatment efficacy was determined based on: (1) microbial eradication (change from baseline in CFUs of causative pathogens); (2) the physician’s clinical impression of change from baseline in cardinal signs (conjunctival discharge, bulbar conjunctival injection, and palpebral conjunctival injection); and (3) change from baseline in ocular signs (erythema/swelling, corneal epithelial disease, corneal stromal disease, and uveitis) and symptoms (burning/stinging, itching, tearing, foreign body sensation, photophobia, and discomfort). Preverbal children who could not communicate their symptoms were excluded from this analysis. Table 2 summarises the four point rating scales, primary definitions, and terms used to evaluate treatment efficacy.
Table 2.
Terms and definitions used for assessment of treatment efficacy
| Score | Parameter evaluated | Definition | |
| Microbial outcome | Change from baseline culture results | ||
| Resolved | 0 | Absence of baseline organisms, no growth | Eradication |
| Improved | 1 | Reduction below pathogenic criteria | |
| No change | 2 | No response or overall improvement | Non-resolved |
| Worse | 3 | Increase in baseline organisms | |
| Clinical outcome | Change from baseline in cardinal signs | ||
| Resolved | 0 | Absence of cardinal signs | Cure |
| Improved | 1 | At least a 1 unit improvement* | |
| No change | 2 | No overall response | Non-resolved |
| Worse | 3 | At least a 1 unit worsening | |
| Ocular signs and symptoms | Change from baseline | ||
| Resolved | 0 | Absence of ocular sign or symptom | Resolved |
| Improved | 1 | At least a 1 unit improvement | |
| No change | 2 | No overall response | Non-resolved |
| Worse | 3 | At least a 1 unit worsening |
*Based on rating scale described in Table 1.
Safety assessments
Adverse events were coded using a modified Coding Symbols for a Thesaurus of Adverse Reaction Terms (COSTART) dictionary. At each follow up visit, patients were asked about the occurrence of any adverse events since the previous visit. If an adverse event had occurred, a description of the event was recorded, and data were collected concerning onset, severity, treatment required, outcome, and the investigator’s assessment of the probability of the event’s relation to the study medication.
Other safety evaluations included assessment of ocular symptoms, visual acuity tests, slit lamp biomicroscopy, and ophthalmoscopy. Ocular signs and symptoms were graded on a four point (0–3) scale as absent (0), mild (1), moderate (2), or severe (3). A two unit worsening from baseline was considered clinically significant. No assessment of ocular symptoms or visual acuity was obtained for preverbal children.
Best corrected visual acuity was assessed using the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart and the logMAR scoring system. A two point rating scale (0 = normal, 1 = abnormal) was used during fundus examinations to assess the retina, macula, choroid, optic nerve, and vitreous humour.
Statistical methods
Demographic and baseline characteristics
Comparisons between treatment groups were analysed using analysis of variance for age and the Mantel-Haenszel χ2 test for race and sex stratifying by study centre.
Efficacy analysis
The statistical plan of the study protocol stipulated that only patients who had positive cultures at baseline, met clinical entry criteria, had some follow up efficacy data, and had no significant protocol violations were to be included in the efficacy analysis. This restricted the efficacy analysis to those patients who most closely met the requirements of and had data collected according the defined study protocol (that is, the “per protocol” population), and eliminated the inclusion of patients in the efficacy analysis who did not meet entry criteria or for whom lack of data may have confounded interpretation of the results. For subgroup analyses by age, patients were stratified as children (2–11 years of age), adolescents (12–16 years of age), or adults (>16 years of age).
In patients with bilateral bacterial conjunctivitis, only one eye from each patient (that is, the eye with the more severe baseline evaluation scores) was included in the efficacy analysis. “End point” was defined as the last observation made, which may or may not have corresponded to the final planned study visit, depending on whether or not the subject completed all planned follow up visits. Thus, the number of patients who completed the “end point” was greater than the number of subjects who completed a final visit. Differences between treatment groups for microbial eradication and clinical cure rates at each study period were compared using the Mantel-Haenszel χ2 test, stratifying by study centre. Based on the results from previous studies of 0.5% levofloxacin and placebo, this study had an estimated power of at least 90% to detect differences in response rates at the significance level of α = 0.05.
Resolution rates for ocular signs and symptoms included only those patients who had an abnormal baseline value and patients who had a normal baseline value and experienced some change during the study. Change values were calculated for each treatment group and compared using Fisher’s exact test.
Safety analysis
All patients who received at least one dose of study medication and had some follow up data were included in the safety analysis (safety evaluable population). Between group differences in the proportion of patients reporting an adverse event or in changes from baseline in biomicroscopy, ocular symptoms, visual acuity, and fundus examination results were analysed using Fisher’s exact test; if bilateral infection at baseline required the treatment of both eyes, the average changes from baseline were analysed.
Subset analyses were also conducted by age for biomicroscopy, ocular symptoms, visual acuity, and fundus examination results to determine the number of patients in each category (composite score for each parameter) who demonstrated a worsening from baseline at end point.
RESULTS
Patient disposition
A total of 249 patients were enrolled; 126 were randomly assigned to the 0.5% levofloxacin treatment group, and 123 were randomly assigned to receive placebo. Of these, 227 patients completed the study (0.5% levofloxacin, n = 115; placebo, n = 112). Reasons for discontinuation included adverse events (n = 7), lost to follow up (n = 6), non-compliance (n = 4), clinical worsening (n = 3), entry violation (n = 1), and lack of cooperation (n = 1). There were no notable differences in discontinuation rates between treatment groups.
A total of 244 patients comprised the safety evaluable population; 117 patients were included in the per protocol population and evaluated for efficacy; 132 enrolled patients were excluded from the efficacy analysis because of negative baseline clinical or microbial evaluations, absence of post baseline data, or significant protocol violations.
Patient demographics
Patient demographics are summarised in Table 3. There was a significant difference in the distribution of male and female patients in the per protocol population (p = 0.036); in the 0.5% levofloxacin treatment group, 63% of patients were female compared with 44% of placebo treated patients. In the safety evaluable population, females comprised 63% of patients in the 0.5% levofloxacin group and 51% of patients in the placebo group; this difference approached significance (p = 0.058). In both populations, there were no significant differences between treatment groups for age or race.
Table 3.
Patient demographics at baseline
| Safety evaluable population | Per protocol population | |||||
| Levofloxacin | Placebo | p Value* | Levofloxacin | Placebo | p Value* | |
| Patients (n) | 124 | 120 | 60 | 57 | ||
| Age (years) | ||||||
| Mean (SD) | 34.5 (20.2) | 33.8 (21.6) | 0.810 | 31.4 (22.3) | 31.6 (23.0) | 0.959 |
| Range | 2–91 | 2–86 | 2–91 | 2–76 | ||
| Subgroups; n (%) | ||||||
| >16 years | 98 (79.0) | 89 (74.2) | 41 (68.3) | 37 (64.9) | ||
| 12–16 years | 7 (5.6) | 9 (7.5) | 3 (5.0) | 3 (5.3) | ||
| 2–11 years | 19 (15.3) | 22 (18.3) | 16 (26.7) | 17 (29.8) | ||
| Sex: n (%) | ||||||
| Female | 78 (62.9) | 61 (50.8) | 0.058 | 38 (63.3) | 25 (43.9) | 0.036 |
| Male | 46 (37.1) | 59 (49.2) | 22 (36.7) | 32 (56.1) | ||
| Race: n (%) | ||||||
| White | 94 (75.8) | 94 (78.3) | 44 (73.3) | 46 (80.7) | ||
| Black | 30 (24.2) | 26 (21.7) | 10 (16.7) | 6 (10.5) | ||
| Hispanic | 15 (12.1) | 6 (5.0) | 0.258 | 5 (8.3) | 3 (5.3) | 0.654 |
| Asian | 1 (0.8) | 1 (0.8) | 0 (0) | 1 (1.8) | ||
| Other | 2 (1.6) | 1 (0.8) | 1 (1.7) | 1 (1.8) | ||
*Based on analysis of variance for age and Mantel-Haenszel χ2 test for sex and race.
Efficacy
Antimicrobial efficacy
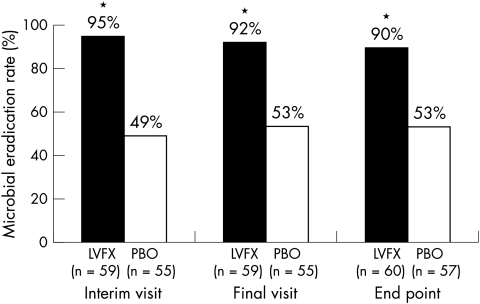
Statistically significant differences in microbial eradication rates in favour of 0.5% levofloxacin treatment were observed at each of the three study visits (Fig 1). At each visit, approximately twice as many patients in the 0.5% levofloxacin group as in the placebo group achieved microbial eradication (p <0.001).
Figure 1.
Microbial eradication rates at the interim visit, final visit, and at end point among patients receiving 0.5% levofloxacin ophthalmic solution (LVFX) or placebo (PBO). *p <0.001 v placebo.
Subgroup analyses by age revealed statistically significant differences in microbial eradication rates at end point in favour of 0.5% levofloxacin in children (2–11 years) and adults. In the 0.5% levofloxacin treatment group, 88% of children achieved microbial eradication, compared with 24% of children receiving placebo (p <0.001). Corresponding microbial eradication rates in adults were 90% v 65%, respectively (p = 0.007). There was no significant difference in microbial eradication rates between treatment groups in the subset of adolescents; however, patient numbers were very small.
Both Gram negative and Gram positive organisms were isolated at baseline. The distribution of pathogens was similar between treatment groups. At baseline, the most commonly isolated organisms at the lowest threshold (1 CFU/ml) were Streptococcus pneumoniae (38%, 44/117 patients) and Haemophilus influenzae (31%, 36/117 patients). Eradication rates by organism from baseline to the final visit are summarised in Table 4. At the final visit, eradication rates for S pneumoniae and H influenzae were higher in the 0.5% levofloxacin treatment group (84% and 92%, respectively) than in the placebo group (47% and 52%, respectively). At both the interim (data not shown) and final visits, all other baseline pathogens were completely eradicated (100%) in the 0.5% levofloxacin group. One of six patients in the placebo group in whom Staphylococcus aureus was isolated at baseline had not achieved microbial eradication at the final visit (Table 4).
Table 4.
Eradication rates by organism from baseline to final visit
| Threshold/organism | 0.5% Levofloxacin No/No (%) | Placebo No/No (%) |
| 1 CFU/ml | ||
| Streptococcus pneumoniae | 21/25 (84) | 9/19 (47) |
| Enterobacter/Pantoea | – | 1/1 (100) |
| Proteus/Morganella | 1/1 (100) | 1/1 (100) |
| Serratia marascens | 1/1 (100) | 1/1 (100) |
| Acinetobacter | 1/1 (100) | 3/3 (100) |
| Haemophilus influenzae | 12/13 (92) | 12/23 (52) |
| Haemophilus parainfluenzae | 2/2 (100) | 3 (2.50) |
| Other Pseudomonas | 4 (3.23) | – |
| Other non-Enterobacteriaceae | ||
| 10 CFU/ml | ||
| Staphylococcus aureus | 11/11 (100) | 5/6 (83) |
| Other Streptococcus | 6/6 (100) | 4/4 (100) |
| (groups D, G; non-grouped; viridans) | ||
| Moraxella catarrhalis | – | 1/1 (100) |
| 100 CFU/ml | ||
| Staphylococcus aureus | 4/4 (100) | 7/7 (100) |
| Other coagulase negative staphylococci | – | 2/2 (100) |
| Micrococcus/Stomatococcus | – | 1/1 (100) |
| 1000 CFU/ml | ||
| Corynebacterium | 3/3 (100) | – |
CFU = colony forming unit.
Clinical efficacy
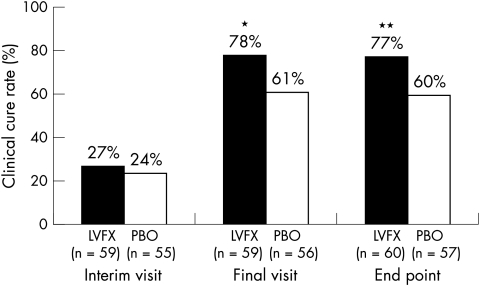
Figure 2 shows that clinical cure rates were significantly greater in the 0.5% levofloxacin treatment group than in the placebo group at both the final visit (p = 0.020) and at end point (p = 0.026). Subgroup analysis by age revealed a significant difference in favour of 0.5% levofloxacin in children; clinical cure rates were 88% and 53% for children receiving 0.5% levofloxacin and placebo, respectively (p = 0.034).
Figure 2.
Clinical cure rates at the interim visit, final visit, and at end point among patients receiving 0.5% levofloxacin ophthalmic solution (LVFX) or placebo (PBO). *p = 0.020 v placebo, **p = 0.026 versus placebo.
Resolution of ocular signs and symptoms
Resolution rates for ocular signs and symptoms were consistently higher in the 0.5% levofloxacin treatment group than in the placebo group at all study visits. The percentages of patients achieving resolution of ocular signs and symptoms at end point are presented in Table 5. Statistically significant differences favouring 0.5% levofloxacin were observed for resolution of the ocular signs of conjunctival discharge (p = 0.027), bulbar conjunctival injection (p = 0.029), and palpebral conjunctival injection (p = 0.018), and for the ocular symptoms of burning/stinging (p = 0.008), itching (p = 0.037), and photophobia (p = 0.023).
Table 5.
Resolution of ocular signs and symptoms at end point
| Patients (%) achieving resolution* | |||
| 0.5% Levofloxacin | Placebo | p Value† | |
| Ocular signs | |||
| Conjunctival discharge | 96.7 | 84.2 | 0.027 |
| Bulbar conjunctival injection | 85.0 | 66.1 | 0.029 |
| Palpebral conjunctival injection | 89.1 | 70.4 | 0.018 |
| Erythema/swelling | 93.6 | 87.5 | 0.672 |
| Ocular symptoms | |||
| Burning/stinging | 91.7 | 67.7 | 0.008 |
| Tearing | 82.3 | 70.0 | 0.167 |
| Itching | 72.9 | 51.0 | 0.037 |
| Foreign body sensation | 84.4 | 72.1 | 0.199 |
| Photophobia | 92.3 | 65.5 | 0.023 |
| Discomfort | 83.7 | 66.7 | 0.085 |
*Excludes those patients who had absence of the sign or symptom at baseline.
†Fisher’s exact test.
Safety
A total of 91 adverse events were reported by 75 patients (31%) in the safety evaluable population during the study. There were no significant differences between treatment groups in the incidence of overall adverse events or treatment related events. Most adverse events were mild to moderate in severity. Conjunctivitis, primarily in the non-study eye, was the most common overall adverse event. Treatment related adverse events (judged by the investigator to be possibly, probably, or definitely related to study medication) were predominantly ocular (as opposed to systemic) and occurred in 9% (11/124) and 6% (7/120) of patients in the 0.5% levofloxacin and placebo treatment groups, respectively. The most common treatment related adverse events in the 0.5% levofloxacin treatment group were transient burning (2.4%) and transient decreased vision (2.4%); however, each of these events occurred in only 3/124 patients. The most common treatment related adverse event in the placebo group was corneal epitheliopathy (2.5%, 3/120 patients). Adverse events reported by at least 2% of patients in either treatment group, regardless of relation to treatment and including those related to the condition being studied, are summarised in Table 6.
Table 6.
Safety evaluation: adverse events*
| 0.5% Levofloxacin (n=124) | Placebo (n=120) | |
| Non-ocular: n (%) | ||
| Headache | 7 (5.65) | 2 (1.67) |
| Pharyngitis | 3 (2.42) | 0 (0.00) |
| Ocular: n (%) | ||
| Conjunctivitis | 18 (14.52) | 12 (10.00) |
| Epitheliopathy | 0 (0.00) | 3 (2.50) |
| Burning | 3 (2.42) | 0 (0.00) |
| Discomfort | 0 (0.00) | 3 (2.50) |
| Decreased vision | 4 (3.23) | 3 (2.50) |
*Reported by at least 2% of patients in either treatment group regardless of relation to study medication.
There were no significant differences between treatment groups in best corrected visual acuity scores, or fundus examination results during the study.
Safety composite scores were analysed to determine the number of patients in specific subgroups who experienced a worsening from baseline at end point. When analysed by age, significantly fewer children in the 0.5% levofloxacin treatment group than in the placebo group experienced a worsening of ocular symptoms (0% v 43%, p = 0.001).
DISCUSSION
The present study demonstrates that a 5 day treatment regimen with 0.5% levofloxacin ophthalmic solution significantly hastens both microbial and clinical cure in patients with bacterial conjunctivitis. At all study visits, microbial eradication was achieved by almost twice as many patients treated with 0.5% levofloxacin as those receiving placebo (p <0.001). Similarly, clinical cure rates were significantly higher with 0.5% levofloxacin than with placebo at both the final visit (p = 0.020) and at end point (p = 0.026). In addition, patients treated with 0.5% levofloxacin achieved significantly faster resolution of most of the ocular signs and symptoms of bacterial conjunctivitis compared to patients who received placebo.
Although there were significantly more women in the 0.5% levofloxacin treatment group than in the placebo group (63.3% v 43.9% in the per protocol population, respectively), it is unlikely that this difference had any bearing on the results of the study. To our knowledge, sex differences in the incidence of bacterial conjunctivitis have not been reported. However, bacterial conjunctivitis is more common in children than adults and often occurs in epidemics.1 In the present study, 28% of enrolled patients were children 2–11 years of age. The microbial eradication rates for this patient subgroup were more than threefold higher in levofloxacin treated patients (88%) than in placebo treated patients (24%, p <0.001). Notably, 0.5% levofloxacin was much more effective than placebo in eradicating S pneumoniae and H influenzae, two of the most common causative organisms in children.14 Clinical cure rates among children were 88% for those treated with 0.5% levofloxacin and 53% for those receiving placebo.
In the United States, children who demonstrate symptoms of conjunctivitis are frequently required to abstain from day care or school because of the concern of disease transmission to other children. The same concerns may apply to adults as well, resulting in lost days of economic productivity. Thus, a 5 day treatment course with levofloxacin 0.5%, which was demonstrated in this study to significantly improve the rates of clinical cure and hasten the eradication of infectious organisms compared to placebo, may have benefits from both a public health and an economic standpoint. Although the pharmacoeconomic implications associated with treatment of bacterial conjunctivitis were beyond the scope of this study, this issue may be an important area for future research.
Earlier studies of older topical fluoroquinolones, such as ciprofloxacin, in the treatment of patients with bacterial conjunctivitis and blepharitis demonstrated eradication or reduction of pathogenic bacteria following a 7 day treatment period.15,16 The efficacy of the 5 day treatment regimen with 0.5% levofloxacin in the present study may also have implications for patient compliance and the development of antibiotic resistance. Premature discontinuation of antibiotic therapy or failure to adhere to the required frequency of administration may increase the risk of treatment failure and lead to prolonged exposure of organisms to subinhibitory concentrations of antibiotic. Thus, the use of an efficacious drug like 0.5% levofloxacin that allows for a shorter, 5 day treatment regimen may improve patient compliance and possibly reduce the risk for creating conditions that might favour selection for antibiotic resistant organisms.
Although S pneumoniae, S aureus, and H influenzae are the most common aetiological pathogens in bacterial conjunctivitis, the disease can be caused by many different organisms.1 Thus, selection of a topical antibiotic with activity against a broad array of potential pathogens would be desirable in designing an empirical antimicrobial regimen for presumed bacterial conjunctivitis. In the present study, 0.5% levofloxacin ophthalmic solution was effective in eradicating a broad spectrum of both Gram negative and Gram positive organisms.
The 5 day treatment regimen with 0.5% levofloxacin ophthalmic solution was well tolerated and demonstrated a safety profile that was similar to placebo; no statistically significant differences were detected between treatment groups in the overall incidence of adverse events and other safety variables. However, a subset analysis of the paediatric population demonstrated some significant differences favouring the administration of levofloxacin 0.5% over placebo. For example, no children (0%) in the 0.5% levofloxacin treatment group experienced a worsening of ocular symptoms, compared with 43% of those who received placebo (p = 0.001).
In parallel with the current study, a second phase III study of 0.5% levofloxacin treatment of bacterial conjunctivitis treatment was recently conducted.17 This study utilised an antibiotic dosing regimen identical to that employed in the current study, but instead of incorporating a placebo comparison group, an active treatment comparison group (0.3% ofloxacin ophthalmic solution) was used. The results from this study showed that a 5 day treatment regimen with 0.5% levofloxacin achieved microbial eradication rates that were statistically superior to those attained with 0.3% ofloxacin.17 Despite the higher concentration of active drug in 0.5% levofloxacin compared with 0.3% ofloxacin, no differences between treatment groups were observed in the incidence of treatment related adverse events.17
In summary, the present study demonstrates that a 5 day treatment regimen with 0.5% levofloxacin ophthalmic solution is safe and effective for the treatment of bacterial conjunctivitis in both children and adults. Results from this study support the current consensus view that despite the self limited nature of bacterial conjunctivitis, treatment of presumed bacterial conjunctivitis with topical antibiotics may provide both individual and public health benefits. Compared to placebo, levofloxacin 0.5% demonstrated higher rates of clinical cure and lower rates of symptomatic relapse in children, and faster rates of microbiological cure in both adults and children. A 5 day course of levofloxacin 0.5% for bacterial conjunctivitis may therefore be beneficial in reducing symptomatology and decreasing the potential risk of disease transmission through person to person spread.
Acknowledgments
Financial disclosure: This manuscript describes data derived from a clinical protocol designed and supported by Santen Inc, Napa, California, USA.
Appendix
The Levofloxacin Bacterial Conjunctivitis Placebo-controlled Study Group
David G Hwang, MD, Richard Casey, MD, Donald Cerise, MD, Luther Crabb, MD, S Lance Forstot, MD, Gary Foulks, MD, Graham Kretchman, MD, Jim Montgomery, MD, Michael Raizman, MD, Michael Rotberg, MD, Jay Rubin, MD, David Schanzlin, MD, David Schulman, MD, Mark Terry, MD, Peter Zloty, MD.
REFERENCES
- 1.Hwang DG. Bacterial conjunctivitis. In: Pepose JS, Holland GN, Wilhelmus KR, eds. Ocular infection and immunity. St Louis: Mosby-Year Book, 1996:799–817.
- 2.Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis; a systematic review. Br J Gen Pract 2001;51:473–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa GS, Hyndiuk RA. The fluoroquinolones: new antibiotics in ophthalmology. Int Ophthalmol Clin 1993;33:59–68. [DOI] [PubMed] [Google Scholar]
- 4.Drlica K. Mechanism of fluoroquinolone action. Curr Opin Microbiol 1999;2:504–8. [DOI] [PubMed] [Google Scholar]
- 5.Ernst ME, Ernst EJ, Klepser ME. Levofloxacin and trovafloxacin: the next generation of fluoroquinolones? Am J Health Syst Pharm 1997;54:2569–84. [DOI] [PubMed] [Google Scholar]
- 6.Morrissey I, Hoshino K, Sato K, et al. Mechanism of differential activities of ofloxacin enantiomers. Antimicrob Agents Chemother 1996;40:1775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RW. Pharmaceutical Research Institute. Data on file. Raritan, NJ, 1997.
- 8.Pickerill KE, Paladino JA, Schentag JJ. Comparison of the fluoroquinolones based on pharmacokinetic and pharmacodynamic parameters. Pharmacotherapy 2000;20:417–28. [DOI] [PubMed] [Google Scholar]
- 9.North DS, Fish DN, Redington JJ. Levofloxacin, a second-generation fluoroquinolone. Pharmacotherapy 1998;18:915–35. [PubMed] [Google Scholar]
- 10.King DE, Malone R, Lilley SH. New classification and update on the quinolone antibiotics. Am Fam Physician 2000;61:2741–8. [PubMed] [Google Scholar]
- 11.Graves A, Henry M, O’Brien TP, et al. In vitro susceptibilities of bacterial ocular isolates. Cornea 2001;20:301–5. [DOI] [PubMed] [Google Scholar]
- 12.QUIXIN [package insert]. In: Sifton DW, ed. Physicians’ desk reference. Montvale: Medical Economics Company Inc, 2001:2872–3.
- 13.Cagle G, Davis S, Rosenthal A, et al. Topical tobramycin and gentamicin in the treatment of ocular infections: a multicenter study. Curr Eye Res February1981;1:523–34. [DOI] [PubMed]
- 14.Soukiasian SH, Baum J. Bacterial conjunctivitis. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea: fundamentals of cornea and external disease. Vol II, Chapter 63. St Louis: Mosby-Year Book, 1997:759–72.
- 15.Adenis JP, Colin J, Verin P, et al. Ciprofloxacin ophthalmic solution in the treatment of conjunctivitis and blepharitis: a comparison with fusidic acid. Eur J Ophthalmol 1996;6:368–74. [DOI] [PubMed] [Google Scholar]
- 16.Bloom PA, Leeming JP, Power W, et al. Topical ciprofloxacin in the treatment of blepharitis and blepharoconjunctivitis. Eur J Ophthalmol 1994;4:6–12. [DOI] [PubMed] [Google Scholar]
- 17.Schwab IR, Friedlaender M, McCulley J, et al. A phase III clinical trial of 0.5% levofloxacin ophthalmic solution versus 0.3% ofloxacin ophthalmic solution for the treatment of bacterial conjunctivitis. Ophthalmology 2003;110:457–65. [DOI] [PubMed] [Google Scholar]