Abstract
Aim: To elucidate the optical basis for unilateral high myopia and to identify the factors associated with its development.
Methods: Medical records of 48 children (aged 4 months to 17 years; mean age 6.8 years) with unilateral high myopia (5 dioptres or more) seen consecutively by the author during a 15 year period were reviewed. 45 (94%) of the 48 patients had unilateral axial myopia.
Results: The mean refractive difference between paired eyes was 9.4 (SD 3.6) dioptres and the more myopic eye was on average 3.3 (1.8) mm longer than the less myopic eye. All but three of the patients had an ocular disorder associated with reduced acuity, central nervous system abnormality, or family history of high myopia.
Conclusion: Clinical conditions associated with unilateral high myopia can be identified in the majority of patients and often account for the associated visual impairment.
Keywords: high myopia, visual outcomes
Unilateral high myopia is an uncommon condition, unlike myopia in general which is found in 15% of children by age 15 years.1–3 Although the eye is too long for its optical power in myopia, the two eyes typically grow by the same amount resulting in symmetric myopia. The presence of asymmetric myopia indicates that either the axial growth or the combined refractive power of the cornea and lens is different between eyes. Axial and refractive myopia can be distinguished on the basis of measurements of corneal power and total axial length. Few published reports have addressed the optical basis for anisometropic myopia derived from these measurements. Sorsby et al inferred from measurements of refractive power in 68 patients that increased axial length was the major cause of anisometropic myopia.4
Most other studies of anisometropic myopia have focused on treatment and visual outcomes.5–7 Clinical findings have not been emphasised except by Rosenthal et al who reported fundus abnormalities (staphyloma and pigmentary disturbances) or cataract in seven of 53 patients.8 But conversely, numerous studies have stressed the increased incidence of axial myopia in monocularly deprived animals9–14 and in human infants with the unilateral presence of blepharoptosis, corneal opacification, cataract, or vitreous haemorrhage.15–18 A higher incidence has also been reported with medullated nerve fibres and optic nerve hypoplasia.19,20 Whether there is a significant prevalence of these predisposing factors in unselected patients with anisometropic myopia is unknown.
A consecutive series of patients with unilateral high myopia were evaluated with the intent of identifying associated clinical conditions and learning more about the mechanisms that underlie unilateral axial myopia and visual impairment.
PATIENTS AND METHODS
The study sample consisted of all children with unilateral high myopia, seen consecutively over a 15 year period. Unilateral high myopia was defined as relative myopia of at least 5 dioptres with absolute myopia of at least 3.5 dioptres. Since measurements of total axial length (TAL) and corneal power have been a routine part of my examination, the majority of patients with unilateral high myopia were entered into this study. Patients who had undergone cataract removal or scleral buckling procedures were excluded.
All patients had complete eye examinations. Refractions were determined 40 minutes after instilling one or two drops of cyclopentolate. (Cyclopentolate 0.5% was used in infants less than 6 months of age, 1.0% was used in children between the ages of 6 months and 2 years, and 2% was used in older children.) Total axial length (TAL) was measured by A-scan ultrasonography (Ophthascan; Biophysic Medical, Clermont-Ferrand, France). Corneal power was measured with a table mounted keratometer (CLC Ophthalmometer; American Optical, Buffalo, NY, USA), or hand held keratometer (MK 500; Nidek, Tokyo, Japan). All measurements of TAL and corneal power were done in awake children in the outpatient clinic.
Axial myopia was defined as myopia which was due to an interocular difference in TAL. Refractive myopia was defined as myopia which was due to an increased refractive power of the cornea, lens, or both. Axial anisometropic myopia was considered to be absolute when the TAL of the more myopic eye was above the normal range and relative when the TAL of the more myopic eye was within the normal range or below.
Visual acuities were measured using optotypes, Allen cards, or Teller acuity cards (TACs) in 40 patients depending upon age and ability level. To provide a common measure and linear scale, acuities were transformed into the logarithm of the maximal angle of resolution in minutes of arc (logMAR) where 20/20 = 0.0, 20/200 = 1.0, etc. Acuities of 20/2000 or less were arbitrarily assigned a value of 2.0.
Ten patients in this series were treated for amblyopia with occlusion therapy. The remaining 38 patients were not treated for amblyopia because they were visually mature (8 years of age or older) or the underlying ocular abnormality was severe enough to account for the reduced vision.
RESULTS
Forty eight patients (23 males, 25 females) ranging from 4 months to 17 years of age (mean age 6.8 years) satisfied the selection criteria. The more myopic eye was the right eye in 21 patients. Forty five of the 48 patients had axial myopia and three had refractive myopia.
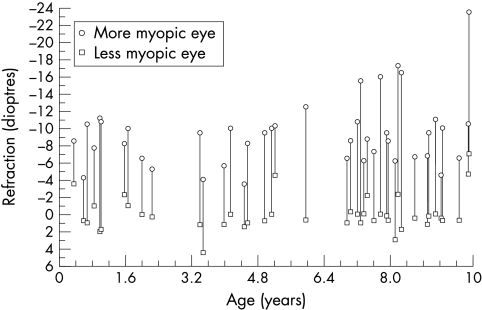
Anisometropic myopia was also categorised on the basis of the refraction in the fellow eye. Twelve of the patients had anisometropic bilateral myopia where both eyes are myopic; 19 had mixed anisometropic myopia where one eye is myopic and the other eye is hyperopic (1 dioptre or more); and 17 had anisometropic unilateral myopia where one eye is myopic and the other eye is emmetropic. Figure 1 shows the bilateral refractions in dioptres for the 42 patients who were aged 10 years or younger.
Figure 1.
Refractions in dioptres for 42 patients with unilateral high myopia. Hyperopic refractions are represented by unsigned values and myopic refractions by minus (–) values.
Twenty seven (56%) of the patients had esotropia (16) or exotropia (11). The remaining 21 patients were orthophoric or had microstrabismus. Binocularity was reduced or absent in all but one patient in whom it was measured.
Corneal power was measured bilaterally in 24 (50%) of the 48 patients. Corneal power (in spherical equivalent) ranged from 40.0 to 49.4 dioptres (mean 44.4 dioptres). The mean (SD) for the interocular difference in corneal power was 0.1 (0.1) dioptres.
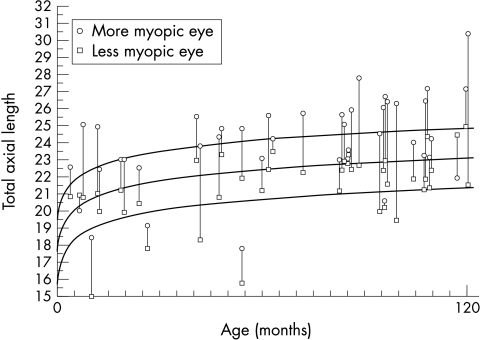
Figure 2 shows the bilateral TAL values in millimetres versus age in months for 42 patients with anisometropic myopia who were age 10 years or younger. The mean values and 95% confidence intervals for age similar normal subjects are shown for comparison.21 For six older children normative values were extrapolated to a mean TAL of 23.8 mm determined by Gordon et al22 (data not shown). Among the 45 patients with axial myopia the more myopic eye was above the upper 95% confidence band in 26 (57.8%) of 45 patients or within the 95% confidence interval in 19 (42.2%) patients. By comparison, the less myopic eye was within the 95% confidence interval in 39 (86.7%) patients or below in six patients. In other words, the anisometropic axial myopia was absolute in 57.8% of patients and relative in the remaining 42.2%.
Figure 2.
Total axial length (TAL) values in 42 patients with unilateral high myopia. For each pair of eyes the circle represents the total axial length of the more myopic eye and the square represents the total axial length of the less myopic eye. The mean total axial length values for normal subjects (middle curve) and upper and lower 95% confidence bands (outer curves) are shown for comparison.
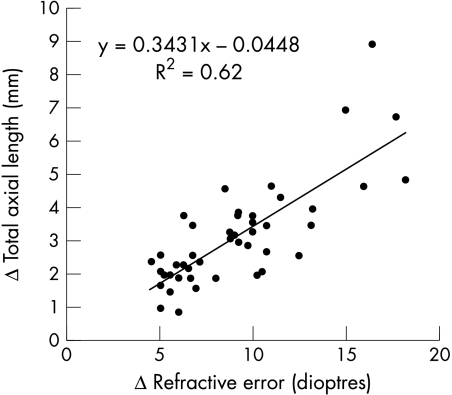
Figure 3 compares the refractive difference in dioptres between eyes with the corresponding difference in TAL in millimetres. The mean refractive difference was 9.4 (SD 3.6) dioptres (range 4.5–18.2 dioptres). The more myopic eye was an average 3.3 (1.8) mm (range 0.8–8.9 mm) longer than the less myopic eye. A slope of 0.34 for the regression line indicates that there is a 2.9 dioptre refractive difference for each 1 mm of TAL difference. Thus, for all patients, the interocular refractive difference was predominantly accounted for by the interocular difference in TAL (r2 = 0.62; p <0.001).
Figure 3.
The refractive difference between the more myopic eye and less myopic eye in dioptres is compared with the difference in their total axial length in millimetres.
Three (6%) of the patients had unilateral refractive myopia associated with lens abnormalities. One of these three patients had a previous corneal laceration complicated by lens subluxation and segmental cataract. A second patient had lens subluxation following blunt ocular trauma. A third patient had a unilateral malformation of the anterior segment with a thickened lens compared to the other eye (7.9 mm versus 4.1 mm). The mean refractive difference between the myopic eye and the less myopic eye was 8.4 dioptres (range 7.5–9.7). In each patient the interocular refractive difference was due to an increase in refractive power of the lens. The mean interocular difference in TAL was 0.1 mm (range 0.0–0.2) and corneal power of both eyes was equal.
Ocular disorders, systemic abnormalities, or relevant family history which were found to be associated with unilateral high myopia are listed in Table 1. Relevant factors were identified in all but three patients. Fifteen (31.3%) patients had optic nerve abnormalities including hypoplasia (10), myelinated nerve fibres (three), atrophy (one), and coloboma (one). Optic nerve hypoplasia was segmental in four patients, diffuse in six patients. Ten (20.8%) of the patients had CNS abnormalities in early life related to perinatal hypoxic ischaemic injury (four), meningitis (two), trauma (two), congenital hydrocephalus (one), or bilateral occipital lobe infarctions (one). Lens abnormalities were implicated in six (12%) patients, three with axial myopia and three with refractive myopia (see preceding paragraph). Of those with axial myopia, two had fetal nuclear cataracts and one had posterior lenticonus. Three (6.6%) of the patients had buphthalmos, two with unilateral or asymmetric glaucoma and one with neurofibromatosis. There was a family history of bilateral high myopia in three patients, one with hemifacial microsomy (case 3) and one with a presumed defect in unilateral emmetropisation (case 1). One of the patients without a known associated condition had nasomaxillary dysplasia (Binder syndrome).
Table 1.
Factors associated with development of unilateral high myopia
| Associated factor | Number of patients (%) |
| Optic nerve disorder | 15 (31.3) |
| CNS abnormality | 10 (20.8) |
| Lens abnormality | 6 (12.5) |
| Retinopathy of prematurity | 5 (10.4) |
| Family history of high myopia | 3 (6.3) |
| Buphthalmos | 3 (6.3) |
| Macular scar/chorioretinal coloboma | 2 (4.1) |
| Congenital ptosis | 1 (2.0) |
| None* | 3 (6.3) |
*One of these patients had nasomaxillary dysplasia (Binder syndrome).
Five patients were born prematurely (mean gestational age 26.6 weeks) and had a history of retinopathy of prematurity (ROP). The TAL of the longer eye was within the normal range in two patients and below in three patients.21 The more myopic eye was on average 2.8 mm (range 1.4–5.3 mm) longer than the less myopic eye. Corneal powers of paired eyes were within 1.5 dioptres of each other. All of the patients had ROP residua in the posterior pole. The relation of anisometropic myopia to total axial length and ROP residua is shown in Table 2.
Table 2.
Relation of anisometropic myopia to visual acuity, total axial length, and ROP residua
| Case | Age (years) | Sex | Eye | Visual acuity | Refraction (dioptres) | TAL (mm) | ROP residua |
| A | 0.8 | F | R | CSM* | −8.50 + 1.50 × 45° | 18.4 | Macular heterotropia |
| L | UCUSUM | −1.50 + 1.00 × 180° | 15.0 | Retinal fold | |||
| B | 2.3 | M | R | CSM | −5.75 + 1.00 × 180° | 19.2 | Normal |
| L | UCUSUM | +0.50 | 17.8 | Macular heterotopia | |||
| C | 4.5 | F | R | UCUSUM | +1.00 + 1.00 × 105° | 15.8 | Retinal fold |
| L | CSM | −4.00 + 1.00 × 90° | 17.8 | Normal | |||
| D | 5.0 | F | R | 5/30 | −9.50 | 23.1 | Retinal fold |
| L | 20/30 | +0.75 | 21.2 | Normal | |||
| E | 3.5 | F | R | 10/30 | −4.00 | 23.8 | Macular heterotopia |
| L | 1/30 | +4.50 | 18.3 | Previous exudative retinal detachment |
*CSM = central, steady, and maintained fixation. UCUSUM = uncentral, unsteady, and unmaintained fixation.
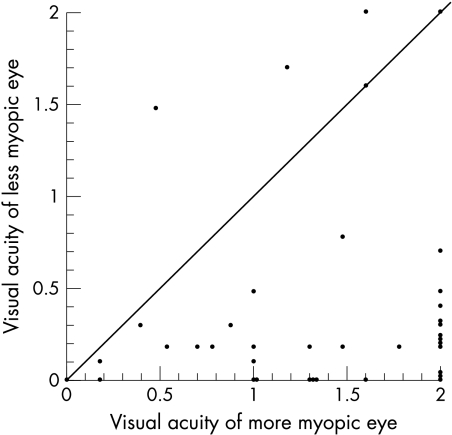
Acuities were bilaterally measured with optical correction in 40 patients using the Snellen test (32), Allen cards (six), or the Teller acuity card procedure (two). Data from the remaining eight patients in whom acuities were not measured objectively were excluded. Figure 4 is a scatter plot of the acuity data. For 34 of 40 patients, acuity is worse in the more myopic eye with 30 of those having acuities between 20/20 and 20/40 (0.0 to 0.3 logMAR) in the less myopic eye and 20/200 to 20/2000 (1.0 to 2.0 logMAR) in the more myopic eye. Five of the six patients in whom the acuity of the more myopic eye was equal to or better than that of the less myopic eye had bilateral abnormalities with probable asymmetric severity of involvement (optic atrophy, chorioretinal coloboma, ROP, or occipital lobe infarction). The one patient with 20/20 acuity bilaterally had a normal eye examination (case 3).
Figure 4.
Visual acuity of the more myopic eye is compared with the visual acuity of the less myopic eye in 40 patients. Visual acuity is expressed as the logarithm of the maximal angle of resolution (logMAR). Acuities of 20/2000 or less were arbitrarily assigned log MAR values of 2.0. The diagonal line represents the total distribution of interocularly identical acuities. Note that single circles located on the x-axis at the value 2, and on the y-axis at 0.1 and 0.4 represent two pair of patients with identical acuities.
The following case reports are presented because each implicates a novel mechanism associated with asymmetric eye growth.
Case 1
A 2 month old girl enrolled in a longitudinal study of visual development was noted to have anisometropic hyperopia (+0.50 + 0.50 × 60 right eye; +3.75 + 0.75 × 130 left eye). Visual acuity was 20/130 right eye and 20/180 left eye using TACs. Examination of the anterior and posterior segments was normal. Family history revealed that father had high myopia and mother acquired mild myopia in her late teens. At 5 months visual acuity was 20/130 right eye and 20/360 left eye and the refraction was the same. The child was prescribed her full hyperopic correction to treat amblyopia left eye and to maximise binocular visual development. Repeat refraction at 8 months was −0.50 + 0.25 × 90 right eye and −4.25 left eye. Visual acuity was 20/94 right eye and 20/130 left eye. Measurements of TAL were 20.1 mm right eye and 20.9 mm left eye. Follow up at 21 months revealed visual acuity 20/32 right eye; 20/63 left eye with spectacles and part time occlusion right eye (3 hours/day). Refraction was +1.00 + 0.25 × 90 right eye −4.00 + 0.50 × 180 left eye. TAL values were 21.4 mm right eye and 22.3 mm left eye.
Comment
Unilateral high myopia was presumably due to a primary defect of emmetropisation in one eye.
CASE 2
A previously healthy 6 month old child was initially evaluated for a bilateral occipital lobe infarction following blunt head trauma. Falling between two bedposts, the infant crushed his head but there were no intraocular haemorrhages suggestive of non-accidental trauma. Serial brain computed tomograph (CT) scans showed progression of bilateral occipital lobe atrophy. Estimated visual acuity was light perception only. Refraction at age 20 months was + 2.00 + 100 at 90° right eye and −9.00 left eye. Fundus examination was normal without evidence of optic atrophy. TAL values were approximately 21.0 mm right eye and 24.9 mm left eye. Pattern reversal VEPs were severely reduced and delayed bilaterally. There was no family history of high myopia.
Comment
The development of unilateral high myopia following a bilateral occipital infarction implicates central brain mechanisms in the control of ocular growth.
Case 3
A healthy 13 year old child with congenital esotropia was referred for evaluation of residual esotropia and anisometropic myopia. She was born at term; growth and development were normal. Family history was strongly positive for high myopia. Best corrected acuity was 20/20 in both eyes. External examination revealed right hemifacial microsomia with microtia. There was no preauricular tag or ocular dermoid. Refraction was −1.00 +1.00 at 85° right eye and −6.00 +1.25 at 90° left eye. Motility examination revealed a 40 dioptre esotropia. Slit lamp examination was normal. Fundus examination showed pigmentary changes consistent with axial myopia in the left eye but was otherwise normal. TAL values were 22.9 mm right eye and 24.5 left eye. Keratometry readings were 44.00 by 45.75 bilaterally.
Comment
Unilateral high myopia in a child with a strong family history of bilateral high myopia suggests that hemifacial microsomia may have offset the genetic propensity of the ipsilateral eye to grow excessively.
Case 4
An 8 year old boy was seen in consultation for an abnormal appearing left optic disc with reduced acuity. He was born at term, and development was age appropriate. A maternal great grandmother had bilateral high myopia. Acuity was 20/20 right eye and 20/400 left eye. Refraction was +0.50 right eye and −6.62 left eye. There was a relative left afferent pupillary defect. Fundus examination demonstrated segmental optic nerve hypoplasia left eye and normal sized right optic disc (see Fig 5). TAL values were 21.9 mm right eye and 24.1 mm left eye. Keratometry readings were 45.0 dioptres bilaterally.
Figure 5.
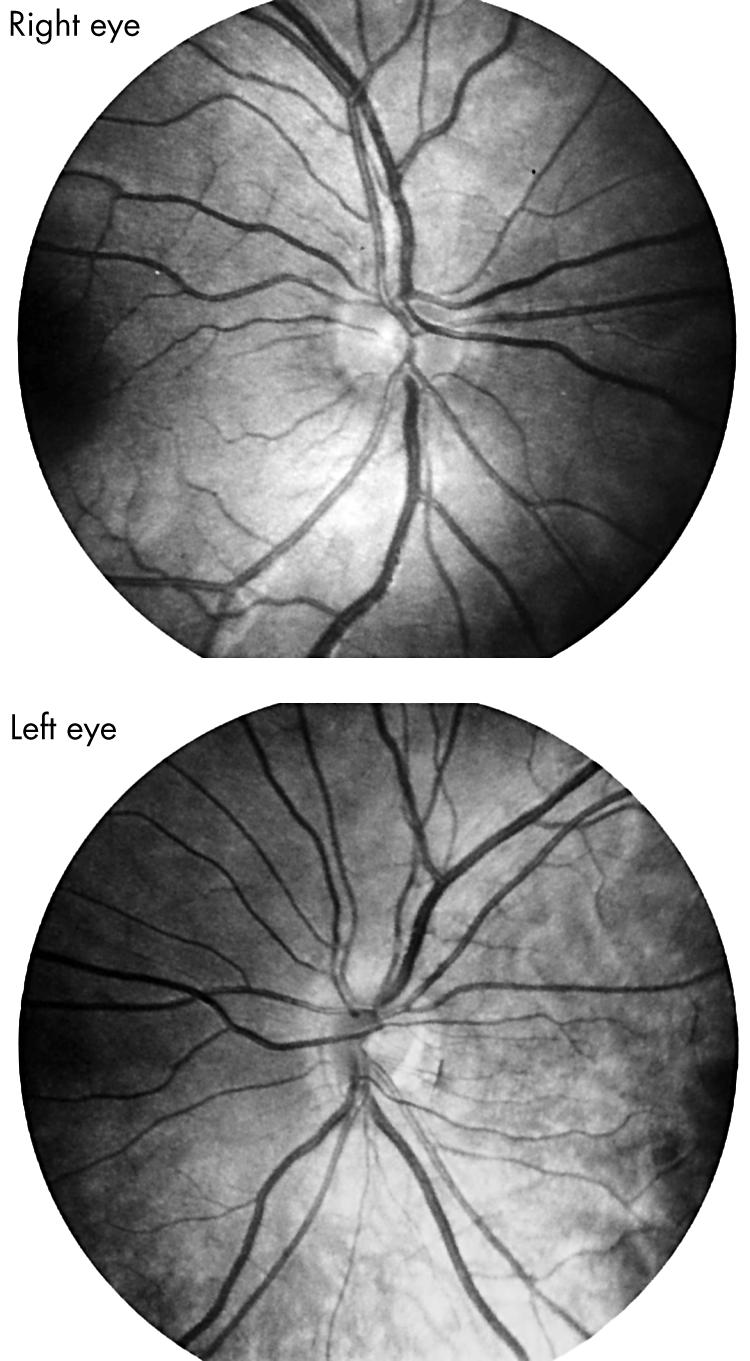
Fundus photographs of an 8 year old boy with unilateral high myopia due to segmental optic nerve hypoplasia left eye. Note that the inferotemporal portion of the left optic disc is truncated and separated from the adjacent pigment epithelium by a crescent of peripapillary tissue.
Comment
The association of optic nerve hypoplasia with unilateral high myopia implicates local retinal and/or central brain mechanisms in the control of ocular growth.
DISCUSSION
We found that 94% of children with unilateral high myopia (5 dioptres or more) had axial elongation of the more myopic eye as the cause of myopic anisometropia confirming the results of Sorsby.4 Comparing the TAL of the more myopic eye with age matched normative data, we found that the anisometropia was absolute in 57.8% of patients and relative in the remaining 42.2%. Only three (6%) of the patients had unilateral high myopia on a refractive basis. Two of the three patients had lens subluxation following blunt trauma. None of these patients had unilateral high myopia resulting primarily from asymmetric increases in corneal power. Excluding patients with eye trauma, all but one patient had unilateral high myopia due to axial length differences.
The higher prevalence of high myopia in children born to highly myopic parents and Mendelian inheritance pattern in some families with high myopia suggest there is an underlying genetic defect in this condition.23–27 Why a genetic defect in the growth plan of the eye would lead to unilateral high myopia is puzzling. One possible explanation for the dissimilar growth between eyes is skewed lyonisation in females heterozygous for X linked myopia. Significantly, each of the three patients in this series with a family history of high myopia were females. Selective inactivation of one parental chromosome in each eye progenitor may lead to predominant expression of the myopia gene in one eye but not in the fellow eye. Precedence for skewed X chromosome inactivation in the eye can be found in monozygotic twins and eye pairs of individuals discordant for colour vision deficiency.28,29 Alternatively, local environmental factors may be important. For example the patient (case 3) with unilateral hemifacial microsomy may have inherited the propensity to high myopia bilaterally but the facial deformation may have influenced the growth of the ipsilateral eye, thereby cancelling the myopia.
Beginning with the work of Wiesel and Raviola on axial myopia induced by form deprivation, environmental factors have been shown to influence how the eye grows. Experimental animals deprived of form vision by suturing the eyelid shut or optical defocusing and human counterparts with ptosis, media opacities can develop ipsilateral axial myopia.9–18 Reduction in the quality of visual inputs presumably provides a weak feedback signal to the central growth control mechanism, thereby resulting in excessive axial elongation under certain conditions. That over 40% of our patients had an obstruction within the visual axis (ptosis, cataract) or anatomic defect of the lens, optic nerve, or macula implicates a vision dependent mechanism.
Although the results presented here focus on the relation between monocular visual deprivation and axial myopia, degradation of visual input is also associated with axial hyperopia.30,31 These data suggest that the sign of the induced refractive error is influenced by severity of visual deprivation and postnatal age. For example, Bradley et al31 have shown in rhesus monkeys that treatment of one eye with a diffuser contact lens retards the growth of the treated eye. In this experiment, the diffuser lens results in a loss of contrast sensitivities across all spatial frequencies relative to severe form deprivation, which differentially degrades high spatial frequency inputs. Moreover, this study highlights the impact of age by showing that visual deprivation from birth induces axial hyperopia. In comparison, unilateral form deprivation during the infantile phase of ocular growth9–18 or adolescence tends to be associated with excessive axial elongation.32
It is important to mention that non-visually dependent mechanisms may be involved in the regulation of ocular growth. Local post-receptoral mechanisms seem to be important. For example, with disorders of the optic nerve and macula the loss of ganglion cells or other retinal cell types have been implicated in the disturbance of local growth mechanisms.33–35
A large percentage (31%) of patients with anisometropic myopia had abnormalities of the optic nerve of which hypoplasia was the most frequent. The high prevalence of unilateral optic nerve hypoplasia in this study compared to previous studies suggests that in the presence of high axial myopia, the concurrent presence of optic nerve hypoplasia is underappreciated. Distinguishing the hypoplastic disc from the optic disc distorted by high myopia can be difficult, especially when the optic disc margin is poorly demarcated. Furthermore, the hypoplastic disc appears larger when viewed through the ophthalmoscope, owing to the magnification effects of high myopia.36,37 Segmental hypoplasia of the temporal optic disc can mimic the tilted optic disc if the peripapillary crescent is mistaken for the temporal portion of the nerve. The observation of high axial myopia with segmental hypoplasia confirms that even modest abnormalities of the optic nerve can be a predisposing factor.38
Myelinated nerve fibres deserve special mention because of their frequent occurrence and well known association with axial myopia and refractory amblyopia.19,20 The abnormality of the optic nerve involves more than just ectopic myelinisation beyond the lamina cribrosa. Visual acuity is usually reduced, there is variable presence of a relative afferent pupillary defect, and VEPs are reported to be abnormal. These visual deficits suggest that the nerve axons and/or visual connections are functionally defective. During development axon signals induce progenitor cells invading the optic nerve to differentiate into type 2 astrocytes or oligodendrocytes.39–41 Developmental abnormalities of nerve axons may lead to faulty differentiation of these precursor cells into oligodendrocytes resulting in ectopic production of myelin within the retinal ganglion cell layer. Myelinated nerve fibres are probably an epiphenomenon caused by the same axonal abnormality that underlies reduced visual function and leads to axial myopia.
Central nervous system abnormalities were the most frequent predisposing factor after optic nerve abnormalities. This association suggests the central nervous system influences eye growth but the literature on this subject is sparse. Raviola and Wiesel hypothesised that striate cortex receives visual inputs and provides feedback signals to subcortical visual mechanisms which, in turn, exert control over eye growth. However, in monkeys, surgical removal of both occipital lobes had no abortive effect on lid suture induced myopia, and pharmacological paralysis of accommodation (with atropine) or stimulation (with isofluorophate) had an inconsistent effect that was species dependent. Despite these inconsistencies, they concluded that axial myopia is caused by an alteration of visual inputs and mediated by the nervous system. By the same mechanism, distortion of visual inputs by the CNS abnormalities described in our study may lead to axial myopia.
One patient (case 1) with longitudinal data provided important insights into the mechanism that regulates emmetropisation during visual development. Initially this infant was found to have anisometropic hyperopia in the left eye (+0.50 right eye + 3.75 left eye) which evolved within a few months without treatment into anisometropic myopia in the left eye (−0.50 right eye −3.50 left eye). One interpretation of this observation is that growth of the left eye lagged initially and then accelerated, overshooting the amount required for emmetropia. That is, there seemed to be a primary defect in the process of emmetropisation in the affected eye.42–44 Why this growth disturbance occurred unilaterally is unclear but the fact that concurrent acuity differences paralleled the refractive differences suggest the underlying mechanism is vision dependent. The same scenario might apply to other cases of “idiopathic” unilateral axial myopia where the eye is otherwise normal and there is no history of visual deprivation.
Congenital glaucoma was readily identified as another predisposing factor in two patients. In the immature eye where the cornea and sclera are still distensible, one eye may differentially elongate due to unilateral or asymmetric increases in intraocular pressure. However, the severity of myopia may not reflect the full amount of axial elongation as the myopia is partially reduced by corneal flattening or posterior displacement of the lens. The possibility that corneal haze or glaucomatous optic nerve damage predisposed to axial elongation on the basis of degraded visual inputs can not be excluded.
Anisometropic myopia is a common sequela of ROP.45 The incidence of anisometropia (defined as an interocular refractive difference of 2 dioptres or more in spherical equivalence) in the CRYO-ROP study was as high as 49% (57 of 117) among the subset of infants with myopia and active ROP in zone 2 (stage 3) or zone 1. Previous studies including axial length measurements have reported shortened, normal or elongated lengths of ROP eyes.46–48 Since TAL is usually normal or abnormally short in ROP eyes, the myopia has been primarily attributed to increases in lens power.49 For the five patients with ROP in this series the more myopic eye was normal size or small but still longer than the fellow eye, indicating that TAL difference was the principal cause of anisometropic myopia. Thus, the axial elongation of the more myopic eye was only relative to the fellow eye which was abnormally small and included ROP residue in four of five patients. Taken together these findings suggest that the severe anisometropic myopia sometimes associated with ROP is primarily due to the reduced growth of the less myopic eye. Furthermore, the more myopic and longer eye tended to have the better visual acuity and more normal appearing macula.
Similar to previous studies which reported visual acuities of 20/100 or worse in 50–85% of patients with anisometropic myopia,6–8 75% of the patients in this study had acuities of 20/200 or worse in the affected eye. Reduced acuities were usually attributed to anisometropic amblyopia and coexisting ocular abnormalities.5–8,50 Since the mean age of our patients (7 years) placed them close to visual maturity, this study cannot adequately address the potential benefits of spectacle correction and amblyopia treatment. Pollard et al reported visual acuities of 20/40 or better in 22 of 40 patients with unilateral high myopia after amblyopia treatment.5 By comparison other investigators have reported that visual loss is refractory to treatment in the majority of patients.6–8
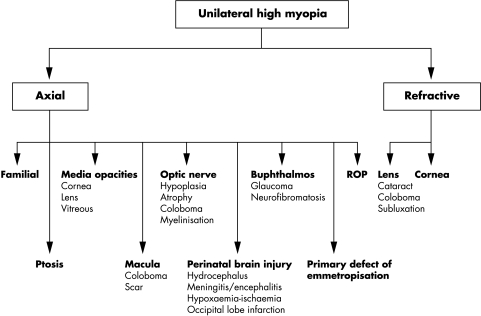
In summary, nearly 90% of the patients with axial myopia had an associated abnormality of the eye, central nervous system, or family history of high myopia. In previous studies, excluding those devoted to specific conditions, little attention has been given to the clinical background in which unilateral high myopia appears. Such a high prevalence of associated pathology in the present series suggests that axial myopia is usually a consequence of a pre-existing abnormality. For patients with a history of a traumatic lens injury, congenital glaucoma, or ROP, the cause for the unilateral high myopia is usually clear. Finding unilateral axial myopia in a patient without a relevant ocular history should prompt the ophthalmologist to look critically for predisposing ocular defects (especially optic nerve disorders) and to carefully consider the perinatal and family history. In an attempt to systematise the diagnostic evaluation of the patient with unilateral high myopia, I propose an algorithm based on my experience (Fig 6).
Figure 6.
Algorithm for diagnostic evaluation of the patient with unilateral high myopia which is based on axial length measurements, clinical findings, and relevant history.
Acknowledgments
Supported in part by an unrestricted grant from Research to Prevent Blindness, Inc, New York, NY, USA
The author has no proprietary interest in any products or companies mentioned in this report.
REFERENCES
- 1.Sperduto RD, Siegel D, Roberts J, et al. Prevalence of myopia in the United States. Arch Ophthalmol 1983;101:405–7. [DOI] [PubMed] [Google Scholar]
- 2.Angle J, Wissman DA. The epidemiology of myopia. Am J Epidemiol 1980;111:220–8. [DOI] [PubMed] [Google Scholar]
- 3.Curtin BJ, ed. Anisometropic myopia. In: The myopias: basic science and clinical management. Philadelphia: Harper and Row, 1985;chapter 17:449–54.
- 4.Sorsby A, Leary GA, Richards MJ. The optical components in anisometropia. Vis Res 1962;2:43–51. [Google Scholar]
- 5.Pollard ZF, Manley D. Long-term results in the treatment of unilateral high myopia with amblyopia. Am J Ophthalmol 1974;78:397–9. [DOI] [PubMed] [Google Scholar]
- 6.Curtin BJ, Schlossman A. Unilateral high myopia in childhood: clinical characteristics and treatment. Am Orthopt J 1976; 26:65–8. [PubMed] [Google Scholar]
- 7.Sanfillipo S, Muchnick RS, Schlossman A. Visual acuity and binocularity in unilateral high myopia. Am J Ophthalmol 1980;90:553–7. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal AR, Von Noorden GK. Clinical findings and therapy in unilateral high myopia associated with amblyopia. Am J Ophthalmol 1971;78:397–9. [DOI] [PubMed] [Google Scholar]
- 9.Wiesel TE, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature 1977;266:66–8. [DOI] [PubMed] [Google Scholar]
- 10.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid sutured tree shrew (Tupaia glis). Brain Res 1977;124:154–7. [DOI] [PubMed] [Google Scholar]
- 11.Wiesel TN, Raviola E. Increase in axial length of the macaque monkey after corneal opacification. Invest Ophthalmol Vis Sci 1979;18:1232–6. [PubMed] [Google Scholar]
- 12.Kirby AW, Sutton L, Weiss AH. Elongation of cat eyes following neonatal lid suture. Invest Ophthalmol Vis Sci 1982;22:274–7. [PubMed] [Google Scholar]
- 13.Raviola E, Wiesel TN. An animal model of myopia. N Engl J Med 1985;312:1609–16. [DOI] [PubMed] [Google Scholar]
- 14.Lauber JK, Oishi T. Lid suture myopia in chicks. Invest Ophthalmol Vis Sci 1987;28:1851–8. [PubMed] [Google Scholar]
- 15.Von Noorden GK, Lewis RA. Ocular axial length in unilateral congenital cataracts and blepharoptosis. Invest Ophthalmol Vis Sci 1987;28:750–2. [PubMed] [Google Scholar]
- 16.Hoyt CS, Stone RD, Fromer C, et al. Monocular axial myopia associated with neonatal eyelid closure in human infants. Am J Ophthalmol 1981;19:197–200. [DOI] [PubMed] [Google Scholar]
- 17.Gee S, Tabbara K. Increase in ocular axial length in patients with corneal opacification. Ophthalmology 1988;95:1276–8. [DOI] [PubMed] [Google Scholar]
- 18.Miller-Meeks MJ, Bennett SR, Keech RV, et al. Myopia induced by vitreous hemorrhage. Am J Ophthalmol 1990;109:199–203. [DOI] [PubMed] [Google Scholar]
- 19.Hittner HM, Antosyzk JH. Unilateral peripapilary myelinated nerve fibers with myopia and/or amblyopia. Arch Ophthalmol 1987;105:943–8. [DOI] [PubMed] [Google Scholar]
- 20.Ellis GS, Frey T, Gouterman RZ. Myelinated nerve fibers, axial myopia and refractory amblyopia: an organic disease. J Pediatr Ophthalmol Strabismus 1987;24:111–19. [DOI] [PubMed] [Google Scholar]
- 21.Weiss AH, Kousseff BG, Ross EA, et al. Simple microphthalmos. Arch Ophthalmol 1989;107:1625–30. [DOI] [PubMed] [Google Scholar]
- 22.Gordon RA, Donzis PB. Refractive development of the human eye. Arch Ophthalmol 1985;103:785–9. [DOI] [PubMed] [Google Scholar]
- 23.Ashton GC. Segregation analysis of ocular refraction and myopia. Hum Hered 1985;35:232–9. [DOI] [PubMed] [Google Scholar]
- 24.Teikari JM, O’Donnell J, Kaprio J, et al. Impact of heredity in myopia. Hum Hered 1991;41:151–6. [DOI] [PubMed] [Google Scholar]
- 25.Zadnik K, Satoriano WA, Mutti DO, et al. The effect of parental history of myopia on children’s eye size. JAMA 1994;271:1323–7. [PubMed] [Google Scholar]
- 26.Young TL, Ronan SM, Drahozal LA, et al. Evidence that a locus for familial high myopia maps to chromosome 18p. Am J Hum Genet 1998;63:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young TL, Ronan SM, Alvear AB, et al. A second locus for familial high myopia maps to chromosome 12q. Am J Hum Genet 1998;63:1419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorgensen AL, Philip J, Raskind WH, et al. Different patterns of inactivation in MZ twins discordant for red-green color-vision deficiency. Am J Hum Genet 1992;51:291–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Graham CH, Hsia Y. Studies of color blindness: a unilaterally dichromatic subject. Proc Natl Acad Sci USA 1959;45:96–9. [Google Scholar]
- 30.Kiorpes L, Wallman J. Does experimentally-induced amblyopia cause hyperopia in monkeys? Vis Res 1995;35:1289–97. [DOI] [PubMed] [Google Scholar]
- 31.Bradley DV, Fernandes A, Tigges M, et al. Diffuser contact lenses retard axial elongation in infant rhesus monkeys. Vis Res 1996;36:509–14. [DOI] [PubMed] [Google Scholar]
- 32.Smith EL III, Bradley DV, Fernandes A, et al. Form deprivation myopia in adolescent monkeys. Optom Vis Sci 1999;76:428–32. [DOI] [PubMed] [Google Scholar]
- 33.Wallman J, Gottleib MD, Rajaram V, et al. Local retinal regions control local eye growth and myopia. Science 1987;237:73–7. [DOI] [PubMed] [Google Scholar]
- 34.Stone RA, Lin T, Laties AM, et al. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA 1989;86:704–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone RA, Laties AM, Raviola E, et al. Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates. Proc Natl Acad Sci USA 1988;85:257–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss AH, Ross EA. Axial myopia in eyes with optic nerve hypoplasia. Graefes Arch Clin Exp Ophthalmol 1992;230:372–7. [DOI] [PubMed] [Google Scholar]
- 37.Lempert P. Optic disc size. Ophthalmology 1996;103:348–9. [DOI] [PubMed] [Google Scholar]
- 38.Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science 1978;201:1249–51. [DOI] [PubMed] [Google Scholar]
- 39.Raff MC, Miller RH, Noble MD. A glial progenitor that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 1983;303:390–6. [DOI] [PubMed] [Google Scholar]
- 40.Raff MC, Abney ER, Fok-Seang J. Reconstitution of a developmental clock in vitro: a critical role for astrocytes in the timing of oligodendrocyte differentiation. Cell 1985;42:61–9. [DOI] [PubMed] [Google Scholar]
- 41.Hart IK, Richardson WD, Bolsover SR, et al. PDGF and intracellular signaling in the timing of oligodendrocyte differentiation. J Cell Biol 1989;109:3411–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabin J, Van Sluyters R, Melach R. Emmetropization: a vision-dependent phenomenon. Invest Ophthalmol Vis Sci 1981;20:561–4. [PubMed] [Google Scholar]
- 43.Judge SJ. Does the eye grow into focus? Nature. 1990;345:477–8. [DOI] [PubMed] [Google Scholar]
- 44.Gwiazda J, Thorn F, Bauer J, et al. Emmetropization. Clin Vis Sci 1993;8:337–44. [Google Scholar]
- 45.Quinn G, Dobson V, Repka M, et al. Development of myopia in infants with birth weights less than 1251 grams. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1992;99:329–40. [DOI] [PubMed] [Google Scholar]
- 46.Fledelius H. Myopia of prematurity: oculometric considerations based upon a Danish material. In: White D, Brown RE, eds. Ultrasound in medicine. Vol 3A. Clinical aspects. New York: Plenum Press, 1977:959–63.
- 47.Tane S, Ito S, Kushiro H, et al. Echographic biometry in myopia of prematurity. Jap J Clin Ophthalmol 1978;32:655–62. [Google Scholar]
- 48.Pulido JS, Byrne SF, Clarkson JG, et al. Evaluation of eyes with advances stages of retinopathy of prematurity using standardized echography. Ophthalmology 1991;98:1099–103. [DOI] [PubMed] [Google Scholar]
- 49.Gordon RA, Donzis PB. Myopia associated with retinopathy of prematurity. Ophthalmology 1986;93:1593–8. [DOI] [PubMed] [Google Scholar]
- 50.Kushner BJ. Functional amblyopia associated with abnormalities of the optic nerve. Arch Ophthalmol 1984;102:683–5. [DOI] [PubMed] [Google Scholar]