Abstract
Aim: To compare incidence of iridial pigmentation prospectively induced by long term treatment with latanoprost and isopropyl unoprostone (hereafter, unoprostone) in Japanese patients with glaucoma.
Methods: Patients with glaucoma treated with prostaglandin (PG) related ophthalmic solutions were sequentially enrolled. Patients treated for more than 30 months with PG related ophthalmic solutions were subjected to analysis. The entry criteria were no history of intraocular surgery, laser iridotomy, and/or laser trabeculoplasty within 12 months before and after the enrolment; and no history of uveitis; no changes in antiglaucoma drugs within 6 months before and after the enrolment. Photographs of the irides were taken under the same conditions and three glaucoma specialists evaluated the iridial pigmentation with masking of patient information. The correlation of iridial pigmentation with the background factors and the reduction of intraocular pressure (IOP) before and after the treatment were investigated.
Results: 48 eyes in 48 patients satisfied the enrolment criteria (25 eyes in the latanoprost group, 23 eyes in the unoprostone group). At the end of the follow up period, iridial pigmentation was present in 15 patients (60.0%) in the latanoprost group and seven patients (30.4%) in the unoprostone group. The correlation between development of iridial pigmentation and age, sex, concurrent use of other ophthalmic solutions, and IOP reduction was not significant.
Conclusions: The incidence of iridial pigmentation induced by latanoprost or unoprostone is high in the case of long term treatment. Iridial pigmentation did not affect PG related ophthalmic solution induced IOP reduction.
Keywords: glaucoma, iridial pigmentation, Japan, latanoprost, isopropyl unoprostone
The currently available prostaglandin (PG) related antiglaucoma ophthalmic solutions in Japan are latanoprost ophthalmic solution (Xalatan, Pharmacia, Uppsala, Sweden) and isopropyl unoprostone ophthalmic solution (hereafter, unoprostone) (Rescula, Ueno Fine Chemical Industry, Osaka, Japan). Clinical studies conducted in the Scandinavia, the United Kingdom, and the United States reported the incidence of latanoprost induced iridial pigmentation,1,2 while the incidence of unoprostone induced iridial pigmentation has been rarely reported because clinical use in the United States and Europe started only in 2000. In Japanese patients, both latanoprost and unoprostone ophthalmic treatments were found to cause iridial pigmentation,3–5 although long term treatment and prospective comparative studies have not been conducted so far.
In the current study, we prospectively investigated the incidence of iridial pigmentation in Japanese glaucoma patients treated for a longer period with one of two types of PG related ophthalmic solutions.
METHODS
The study was conducted in compliance with the Declaration of Helsinki. All patients enrolled as subjects gave consent to participate in the study. At the time of initial evaluation of an adverse event, such as iridial pigmentation, the patients voluntarily consented to continue ophthalmic treatment.
Subject and entry criteria
All patients with glaucoma treated with PG related ophthalmic solutions were sequentially enrolled in the glaucoma clinic of the University of Yamanashi Hospital. Patients treated for more than 30 months with PG related ophthalmic solutions were subjected to analysis. The entry criteria were no history of intraocular surgery, laser iridotomy, and/or laser trabeculoplasty within 12 months before and after enrolment.
The eyes to be studied were chosen randomly in patients in whom both eyes were treated, whereas in the patients receiving unilateral treatment, the treated eye was selected for the study.
Photography and assessment of iridial pigmentation
The iris was observed using an identical slit lamp microscope (FS-3V, Nikon, Tokyo, Japan) before and after ophthalmic treatment. As previously reported,2 under identical conditions (illumination at 45° from the right side of the patient, 16× magnification), the iris was photographed using the same type of slide film (DYNA EX ISO400, Eastman Kodak, Rochester, NY, USA).
The photographs were evaluated by displaying magnified views of two slides separately from the same type of projector (Master HILUX-ZOOM210, Rikagaku, Tokyo, Japan) on a single screen, and three glaucoma specialists individually determined whether there was any progression of the pigmentation, while patient information was masked. For all these specialists, the patient information and the duration of treatment were masked, and the slide films were selected and projected randomly. Patients with photographs that were judged to be indistinct and not evaluable were excluded from the study.
The pigmentation was classified into three levels of “no increase in pigmentation,” “photographic evaluation indeterminate,” and “increased pigmentation.” If there was agreement among all three specialists regarding the assessment of increased pigmentation, the patient was assessed as having “positive pigmentation.”
Confirmation of reliability of assessment
To evaluate the reliability of the assessment procedure, two photographs were taken of each of the arbitrarily selected eyes of eight healthy subjects. The photographs were displayed on the screen as was done for photographs of patients treated with PG related ophthalmic solution, and the three specialists assessed the iridial pigmentation with masking of patient information.
Other end points
The influence of patient disease type, age, sex, and concurrent use of other ophthalmic solutions on the development of pigmentation was studied. For the eyes with pigmentation and the unaffected eyes, the degree of ocular hypotensive effect was determined from the IOP before the start of the PG related ophthalmic treatment and the IOP at the final assessment.
Statistical analysis
Statistical analysis was performed using Fisher’s exact probability test or the unpaired Student’s t test. A significant difference was defined as p <0.05. Results are expressed as means (SD).
RESULTS
Patient profile
In all, 137 patients, including 69 patients treated with latanoprost and 68 patients treated with unoprostone, fulfilled the entry criteria and were enrolled in this study. In all patients, the iris colour was brown. Of these patients, 25 in the latanoprost treatment group and 23 in the unoprostone treatment group completed the study. Detailed patient information and causes of dropout are summarised in Tables 1 and 2, respectively.
Table 1.
Enrolled patient profile
| Latanoprost | Unoprostone | |
| Cases (eyes) | 25 | 23 |
| Sex | ||
| Male:female | 15:10 | 10:13 |
| Age (years) (SD) | 65.0 (12.9) | 69.6 (11.4) |
| Follow up period (months) (SD) | 33.4 (1.8) | 32.9 (3.9) |
| Type of glaucoma: eyes (%) | ||
| POAG | 18 (72.0) | 16 (69.6) |
| NTG | 3 (12.0) | 3 (13.0) |
| Capsular glaucoma | 2 (8.0) | 2 (8.7) |
| PACG | 1 (4.0) | 0 |
| Ocular hypertension | 0 | 1 (4.3) |
| SACG | 1 (4.0) | 1 (4.3) |
POAG = primary open angle glaucoma, NTG = normal tension glaucoma, PACG = primary angle closure glaucoma, SACG = secondary angle closure glaucoma, unoprostone = isopropyl unoprostone.
Table 2.
Causes of dropout (no of eyes, (%))
| Latanoprost | Unoprostone | |
| Poor photographic quality | 7/69 (10.1) | 13/68 (19.1) |
| Change in medication | 16/69 (23.2) | 10/68 (14.7) |
| No show up or change in hospital | 9/68 (13.0) | 13/68 (19.1) |
| Surgical treatment | 12/69 (17.4) | 9/68 (13.2) |
| Total | 44/69 (63.7) | 45/68 (66.2) |
The latanoprost group included 15 men and 10 women, with the mean age being 65.0 (12.9) years (range 35–89 years). Primary open angle glaucoma (POAG) was observed in 18 patients (72.0%); normal tension glaucoma (NTG) in three patients (12.0%); capsular glaucoma in two patients (8.0%); primary angle closure glaucoma (PACG) in one patient (4.0%); and secondary angle closure glaucoma (SACG) in one patient (4.0%).
The unoprostone group included 10 men and 13 women, with the mean age being 69.6 (11.4) years (range 44–89 years). POAG was observed in 16 patients (69.6%); NTG in three patients (13.0%); capsular glaucoma in two patients (8.7%); ocular hypertension in one patient (4.3%); and SACG in one patient (4.3%). The follow up period was 33.4 (1.8) months in the latanoprost group and 32.9 (3.9) months in the unoprostone group. There were no significant differences between the two groups.
None of the patients who developed iridial pigmentation declined the continuation of ophthalmic treatment in the current study.
Concurrent ophthalmic treatment
The concurrent use of multiple ophthalmic solutions is shown in Table 3. In the latanoprost group, three patients (12.0%) received monotherapy, while in the unoprostone group seven patients (30.4%) received monotherapy. There were no significant differences in the distribution of concurrently used ophthalmic solutions between the two groups.
Table 3.
Concomitant use of antiglaucoma eyedrops (no of eyes (%))
| Latanoprost | Unoprostone | |
| Monotherapy | 3 (12.0) | 7 (30.4) |
| + β blocker | 10 (40) | 0 |
| + β blocker, DPE, pilocarpine | 5 (20) | 7 (30.4) |
| + β blocker, DPE | 2 (8.0) | 5 (21.7) |
| + β blocker, pilocarpine | 1 (4.0) | 4 (17.4) |
| + β blocker, dorzolamide | 1 (4.0) | 0 |
| + β blocker, DPE, dorzolamide | 2 (8.0) | 0 |
| + β blocker, DPE, dorzolamide, pilocarpine | 1 (4.0) | 0 |
DPE = dipivefrin.
Consistency in photographic assessment
Healthy volunteers
Regarding the determination of iridial pigmentation in healthy subjects by the specialists, the conclusion was “no increase in pigmentation,” and there was 100% agreement among them.
Glaucoma patients
Overall, there was 75.1% agreement in the determinations made by the three specialists. The agreement was 76% in the latanoprost group and 74.1% in the unoprostone group, with no significant difference between the two groups.
Patients with increased pigmentation
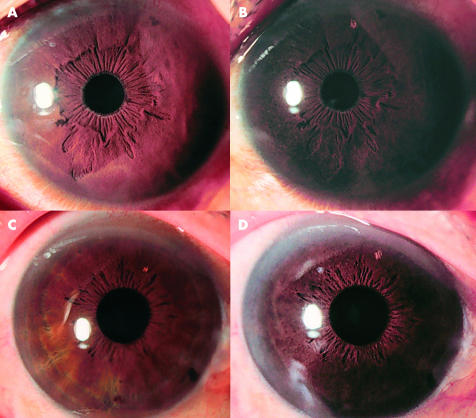
Figure 1 shows the results for a patient who was rated as having increased pigmentation by the three specialists. For both PG agents, the patterns of increased pigmentation were similar, in which one type showed scattered pigmentation in the early stages that gradually increased with progression, and the other type showed pigmentation that occurred throughout with the annulus iridis major as the centre. There was no difference in the pattern of pigmentation between latanoprost and unoprostone.
Figure 1.
Representative case of PG related ophthalmic solution induced iridial pigmentation. Before treatment with PG related ophthalmic solution, iridial pigmentation was normal (A, C). After initiation of treatment, scattered pigmentation was observed and iridial pigmentation gradually increased (B, latanoprost, D, unoprostone). The pattern of increased pigmentation by unoprostone was similar to that by latanoprost.
Incidence of iridial pigmentation in glaucoma patients
At the end of the follow up period, the incidence of positive pigmentation was 60.0% (15/25) in the latanoprost group and 30.4% (7/23) in the unoprostone group. There is a significant difference in the incidence of positive pigmentation between the two groups (p = 0.04).
Factors affecting iridial pigmentation
Table 4 shows the relation between iridial pigmentation and patient age, sex, and concurrent use of other ophthalmic solutions. There were no significant differences in any parameter between the latanoprost group and the unoprostone group. With both PG related ophthalmic solutions, the increased pigmentation tended to be seen more frequently in the elderly, but this trend was not significant.
Table 4.
Factors affecting iridial pigmentation
| Latanoprost | Unoprostone | |||||
| Pigmentation (+) | Pigmentation (−) | p Value | Pigmentation (+) | Pigmentation (−) | p Value | |
| Age (years) (SD) | 67.5 (12.3) | 62.3 (13.4) | 0.33 | 74.7 (8.12) | 66.2 (12.3) | 0.07 |
| Sex (male:female) | 7:6 | 8:4 | 0.69 | 2:5 | 11:5 | 0.17 |
| Concomitant usage (yes:no) | 12:1 | 10:2 | 0.59 | 5:2 | 11:5 | >0.99 |
Fisher’s exact probability test and the unimpaired student’s t test were employed for statistical analysis.
Iridial pigmentation and IOP reduction
In patients with progression of iridial pigmentation, the IOP changed from 19.7 (3.3) mm Hg to 15.8 (2.7) mm Hg (Δ3.9 (1.9) mmHg) in the latanoprost group, and from 19.7 (4.4) mm Hg to 18.3 (2.2) mm Hg (Δ1.33 (5.46) mmHg) in the unoprostone group.
In patients with no progression of iridial pigmentation, the IOP changed from 19.8 (3.8) mm Hg to 16.2 (3.7) mm Hg (Δ3.7 (3.26) mm Hg) in the latanoprost group, and from 21.3 (3.8) mm Hg to 17.9 (3.3) mm Hg (Δ3.4 (3.2) mm Hg) in the unoprostone group.
The extent of changes in the IOP in the unoprostone group was less in the increased iridial pigmentation group compared to the group with no increase in pigmentation, but this difference was not statistically significant.
DISCUSSION
It is important to determine accurately the incidence of iridial pigmentation, which is considered to be probably irreversible or slightly reversible according to Stjrenschants et al,6 with the long term use of antiglaucoma ophthalmic solutions. In this study, in order to analyse accurately the incidence of iridial pigmentation, an observer masked prospective study design was used as in a previous study.2
Clinical studies conducted in the Scandinavia, the United Kingdom, and the United States revealed that after latanoprost ophthalmic treatment for 1 year, the incidence of iridial pigmentation was 11%, 23%, and 12%, respectively, and the incidence was lower in Asians than in white people.1 In Japanese patients treated with latanoprost, the incidence of iridial pigmentation was 24.6% at 12 months and 79.5% at 24 months according to Ogawa and Imai,4 and 26.4% at 6 months and 51.6% at 12 months according to Hara.3 Therefore, the incidence of iridial pigmentation because of latanoprost treatment in the Japanese patient population may be equivalent to or higher than the incidence seen among the white patient population.
With unoprostone, Bee et al7 reported no increase in iridial pigmentation after 52 weeks of administration to cynomolgus monkeys. It was also reported that unoprostone does not cause an increase in iridial pigmentation in Japanese patients.8,9 By contrast, Yamamoto and Kitazawa,5 Iwaguchi et al,10 Oda et al,11 and others reported patients with increased pigmentation. Ogawa and Iwai reported that with long term treatment with unoprostone (mean 5.3 years), the incidence of iridial pigmentation was 2.4%.4 The incidence of pigmentation observed in our study is higher than that reported by Ogawa and Iwai. The reason for this difference is not clear, but may be due to differences in the diagnostic protocol. Assessment of patients with increased pigmentation revealed that those treated with unoprostone had a milder degree of pigmentation increase than those treated with latanoprost, and it is possible that such patients may have been scored as “no change” in the study by Ogawa and Iwai.
In this study, the incidence of pigmentation was significantly higher with latanoprost than with unoprostone treatment, consistent with previous reports.1–4,8,9 The differences in the incidence of iridial pigmentation between latanoprost and unoprostone treatment may be because of differences in the affinity to the PG receptors. The difference in intraocular metabolism may be another reason for this difference in the incidence. The acidic form of latanoprost, an intraocular active form of latanoprost, was reported not to be further metabolised in the eye,12 whereas unoprostone is consequently metabolised by β oxidation and ω oxidation in the eye to its inactive forms within 30 minutes after administration, as found in our previous study.13
The relation between increased iridial pigmentation and age, sex, concurrent use of other ophthalmic solutions, disease type, and extent of ocular hypotension was studied in both groups, and no statistically significant difference was found between them. However, with regard to age, the increased pigmentation tended to be more frequent in the elderly in both groups. The extent of IOP reduction by unoprostone treatment in patients with iridial pigmentation tended to be smaller than that in patients without iridial pigmentation.
It has been reported that with increased iridial pigmentation, there is a corresponding increase in the number of melanin granules in iridial melanocytes, with no effect on the melanocytes or increased proliferation of human iridial melanocyte.1,14,15 It has also been reported that iris melanocytes release melanin via an autoregulatory function and do not release or transport the melanin granules to nearby cells.16,17 Thus, we believe that the iridial pigmentation induced by treatment with PG related ophthalmic solutions is not a major problem, although additional long term studies should be conducted.
Although we took photographs of the iris routinely after the enrolment, for the unoprostone ophthalmic treatment, photographs could not be taken throughout the follow up period under conditions identical to those before the treatment, such as exposure condition, film type, and magnification, so that the incidence of pigmentation over time could not be calculated. Additional studies are needed to address these issues.
REFERENCES
- 1.Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol 1997;41:S129–38. [DOI] [PubMed] [Google Scholar]
- 2.Chiba T, Kashiwagi K, Kogure S, et al. Iridial pigmentation induced by latanoprost ophthalmic solution in Japanese glaucoma patients. J Glaucoma 2001;10:406–10. [DOI] [PubMed] [Google Scholar]
- 3.Hara T. Increased iris pigmentation after use of latanoprost in Japanese brown eyes (in Japanese). Nippon Ganka Gakkai Zasshi 2001;105:314–21. [PubMed] [Google Scholar]
- 4.Ogawa I, Imai K. Iridial pigmentaion and hypertrichosis of eyelids caused by latanoprost instillation (in Japanese). J Eye 2000;17:429–33. [Google Scholar]
- 5.Yamamoto T, Kitazawa Y. Iris-color change developed after topical isopropyl unoprostone treatment. J Glaucoma 1997;6:430–2. [PubMed] [Google Scholar]
- 6.Stjernschantz J, Albert D, Hu D, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol 2002;47:S162. [DOI] [PubMed] [Google Scholar]
- 7.Bee WH, Vogel F, Korte R, et al. Computer-assisted iris color analysis in a 52-week ocular chronic toxicity study on rescula eye-drops in cynomolgus monkeys. Invest Ophthalmol Vis Sci 1997;38:S815. [Google Scholar]
- 8.Azuma I, Masuda K, Kitazawa Y, et al. Double-masked comparative study of UF-021 and timolol ophthalmic solutions in patients with primary open-angle glaucoma or ocular hypertension. Jpn J Ophthalmol 1993;37:514–25. [PubMed] [Google Scholar]
- 9.Yamamoto T, Kitazawa Y, Azuma I, et al. Clinical evaluation of UF-021 (Rescula; isopropyl unoprostone). Surv Ophthalmol 1997;41:S99–103. [DOI] [PubMed] [Google Scholar]
- 10.Iwagichi Y, Tanahashi T, Shirato H. Two cases of Iris color change after instillation of isopropyl unoprostone (in Japanese). Rinsho Ganka 1999;53:971–4. [Google Scholar]
- 11.Oda E, Matsumoto S, Washimi I, et al. Iridial pigmentation possibly caused by isopropyl unoprostone (in Japanese). Ganka 1999;41:1479–83. [Google Scholar]
- 12.Sjoquist B, Basu S, Byding P, et al. The pharmacokinetics of a new antiglaucoma drug, latanoprost, in the rabbit. Drug Metab Dispos 1998;26:745–54. [PubMed] [Google Scholar]
- 13.Kashiwagi K, Iizuka Y, Tsukahara S. Metabolites of isopropyl unoprostone as potential ophthalmic solutions to reduce intraocular pressure in pigmented rabbits. Jpn J Pharmacol 1999;81:56–62. [DOI] [PubMed] [Google Scholar]
- 14.Selen G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Ophthalmol 1997;41:S125–8. [DOI] [PubMed] [Google Scholar]
- 15.Drago F, Marino A, La Manna C. Alpha-methyl-p-tyrosine inhibits latanoprost-induced melanogenesis in vitro. Exp Eye Res 1999;68:85–90. [DOI] [PubMed] [Google Scholar]
- 16.Dieterich C. The fine structure of the melanocytes of the human iris. Albrecht v Graefes Arch Klin Ophthalmol 1972;183:317–33. [DOI] [PubMed] [Google Scholar]
- 17.Prota G, Hu DN, Vincensi MR, et al. Characterization of melanins in human irides and cultured uveal melanocytes from eyes of different colors. Exp Eye Res 1998;67:293–9. [DOI] [PubMed] [Google Scholar]