Abstract
Background/aim: Nestin is an intermediate filament marker for neural progenitor cells. The authors aimed to identify nestin positive cells in adult human retina and within surgically removed epiretinal membranes.
Methods: Adult human retina and epiretinal membranes were studied. Tissue was fixed and processed for semithin sections or whole mount preparations for immunohistochemical detection of nestin and glial fibrillary acidic protein (GFAP) expression.
Results: Nestin positive cells are most prominent at the ora serrata, possess fibrillary processes, small amounts of perinuclear cytoplasm, and are arranged radially within or superficially on the retina. In the posterior retina, speckled cytoplasmic nestin staining is seen around the nuclei of neurons. In the peripapillary retina most of the cells in the retinal ganglion cell layer are nestin positive. These cells appear to represent nestin positive neurons. Speckled cells are also seen in the myelinated portion of the optic nerve. In epiretinal membranes patches of elongated nestin positive cells were found. These cells were also positive for GFAP.
Conclusions: Some neurons and glia in the adult human retina are nestin positive. Their pattern in anterior retina suggests an analogy with the ciliary marginal zone found in many other species. The role of these cells in pathological responses to retinal disease is suggested by the presence of large numbers of ectopic nestin positive cells in epiretinal membranes. The authors hypothesise that nestin positive cells represent a population of progenitor cells from normal adult human retina that differentiate to make up retinal scar tissue.
Keywords: nestin, progenitor cells, neurons, epiretinal membranes, glia, GFAP, retina
Progenitor cells are found in most tissues where they are able to self renew and to differentiate into various phenotypes (multipotential) to generate or regenerate tissues. In the normal adult mammalian central nervous system (CNS) tissue repair and regeneration appear limited owing to a paucity of progenitor cell types or an environment that inhibits (or does not permit) certain patterns of differentiation. Alternatively, regeneration may be ongoing but unseen, except where deficiencies give the appearance of neurodegeneration. Identifying progenitor cells is difficult. Markers of mature phenotype are absent until differentiation, when markers of the undifferentiated state are lost. The pattern of intermediate filament gene expression changes according to cellular status.1 Nestin is a recently characterised class IV intermediate filament expressed in the early CNS2 first described in the neural tube and somites (recognised by the antibody Rat.401).3 Nestin now is increasingly used as a marker for neural progenitor cells.
The two components of the optic vesicle—the inner neural retina and the outer retinal pigment epithelium—are largely developed by the early postnatal period in rodents.4 In the adult mouse eye single pigmented ciliary margin cells have been found which exhibit clonal proliferation in vitro and can differentiate into rod photoreceptors, bipolar neurons, and Müller glia.5–9 These cells proliferate in the presence of basic fibroblast growth factor (bFGF) and express nestin.4 Retinal progenitors derived from adult9 or embryo10 donors proliferate and differentiate when transplanted into models of degenerative diseases. Neonatal or embryonic rat retinal neural progenitor cells proliferate in vitro over 3 weeks, express nestin and differentiate into neurons, glia, or photoreceptors.7 Although neural progenitor cells can be isolated from the human brain after death,11 no reports of such cells having been isolated from the human retina exist.
The importance of this issue lies in the possible role such cells may have in homeostasis of normal tissue and response to injury and disease (where they may display a regenerative faculty or be involved in abnormal healing—that is, scarring). We report on the identification of such cells in the normal human retina and within epiretinal membranes and discuss the implications of our findings in the context of retinal disease.
METHODS
Adult human retina was obtained from donors (to the Corneal Bank, Bristol Eye Hospital) within 24 hours post mortem, with research consent. Epiretinal membranes were obtained from patients undergoing vitreoretinal surgery for a variety of conditions, including proliferative vitreoretinopathy and idiopathic epiretinal membranes. Informed consent was obtained from the patients, and South West Region ethics committee approval was obtained for this work (reference E 4614).
Tissues were fixed in 1% buffered paraformaldehyde. Retinal samples were subsequently immersed in 20% sucrose overnight before embedding in OCT and snap freezing in liquid nitrogen. Sections of 12 μm were cut on a cryostat and air dried onto poly-l-lysine coated glass microscope slides or were processed as whole mounts. Sections were then soaked in phosphate buffered saline (PBS) for 20 minutes, before endogenous tissue peroxidase was neutralised and the sections were washed again. A serum block (10% horse serum, Vector Labs, in 2% bovine serum albumin (BSA) with 0.01% Triton X100) was applied. Primary antibody was applied with 2% BSA at 4°C overnight (negative controls included irrelevant isotype mAb). Primary anti-human nestin (Chemicon) was used at 1 in 200. A biotinylated secondary antibody was applied in 0.1% BSA. Antibodies were visualised using the ABC kit (Vector) followed by a 3,3′-diaminobenzidene reaction. Sections were counterstained with haematoxylin before dehydration, clearing in xylene, and mounting. For immunofluorescence the primary antibody to glial fibrillary acidic protein (GFAP) (Dako) was used at 1 in 1000. Fluorescent secondary antibodies (Jackson Immunochemicals, USA) were used at 1 in 50.
RESULTS
Normal adult human retina
Nestin positive cells were identified in normal human retina by immunocytochemistry. There are at least two morphologically distinct nestin positive cell types in the human retina. The first type of cell displayed speckled cytoplasmic nestin expression (Fig 1). The second cell type was elongated, with filamentous processes and smaller amounts of perinuclear cytoplasm (Fig 2). Occasionally cells had both features (see below). No nestin positive cells were found in the outer retina. These cell types were not evenly distributed in the adult human retina (see below). Specificity to nestin was confirmed by the absence of staining with isotype control mAb.
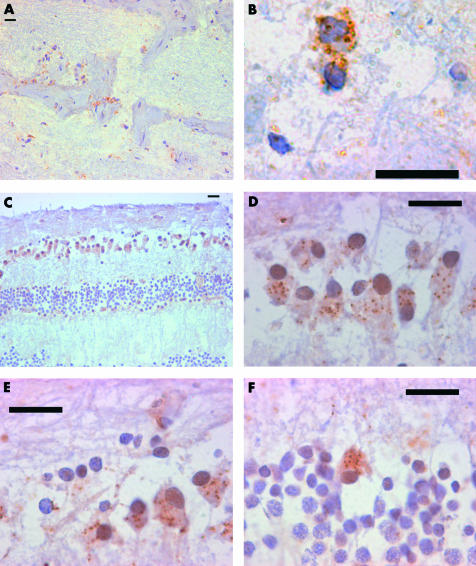
Figure 1.
Speckled nestin positive cell distribution in adult human retina. Composite of nestin immunocytochemistry of adult human retina to the show morphology, extent, and distribution of speckled nestin positive cells in the adult human retina. All scale bars represent 20 μm. (A) The optic nerve contains speckled cells within the myelinated nerve fibre bundles and where the latter meet fibrous septa. (B) A high power view of speckled cytoplasmic staining in a cell in the optic nerve. (C) A cross section of peripapillary retina showing the distribution of nestin immunoreactive cells within the ganglion cell layer (GCL) and inner nuclear layer (INL). (D) Retinal ganglion cell nuclei in the GCL showing speckled cytoplasmic staining. (E) Retinal ganglion cell nuclei in the GCL showing speckled cytoplasmic staining. Here not all retinal ganglion cell nuclei are associated with nestin positive cytoplasm. (F) Nuclei in the INL were sometimes associated with speckled cytoplasmic staining.
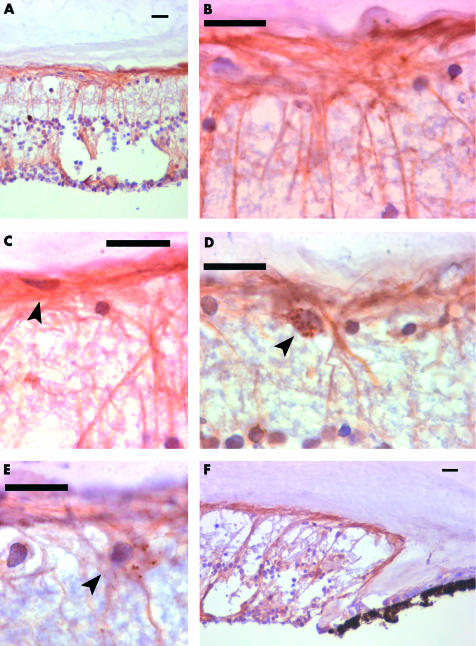
Figure 2.
Nestin positive cells with an elongated morphology in the adult human retina. Composite of nestin immunocytochemistry of adult human retina to the show morphology, extent and distribution of elongated nestin positive cells with fibrous processes in the adult human retina. All scale bars represent 20 μm. (A) The peripheral retina contains large numbers of nestin positive cells, with elongated fibrous processes. Some are radially and others superficially arranged. This section of retina contains an area of cystic retinal change. (B) A high power view of the retinal surface in the peripheral retina. Radial and superficial fibres interlock and are continuous. (C) Some nestin positive cells form the innermost layer of the retina (arrowed). These cells give rise to processes running along the retinal surface. (D) In the anterior retina, some cells are found with speckled cytoplasmic staining (arrowed), in close association with nestin positive fibrous processes. (E) In the peripheral retina some speckled cells (arrowed) are seen to give rise to fibrous processes. (F) Nestin positive cells extend right up to the ora serrata. The cells with fibrous processes extend right up to the ora serrata.
Optic nerve
Posteriorly in the optic nerve, nestin positive cells are found beyond the point where myelination begins (Fig 1A, B). These speckled cells are found associated with bundles of myelinated nerve fibres, within the optic nerve.
Peripapillary retina
The peripapillary retina displayed nestin positive cells in the ganglion cell layer (GCL). These cells have a rounded morphology, and speckled cytoplasmic staining (Fig 1C, D, E). Speckled nestin staining was also noted in the inner nuclear layer (INL) (Fig 1F), similar to those seen in the GCL. In this part of the retina, there was minimal fibrillary staining in the inner limiting membrane (ILM) and nerve fibre layer (NFL).
Posterior and equatorial retina
The posterior and mid-peripheral retina showed only occasional nestin positive cells in the GCL, but these are very infrequent. Occasional fibrillary nestin positive cells were seen in the ILM and NFL.
Peripheral anterior retina
The pattern of nestin staining was dramatically different in the extreme retinal periphery. At the ora serrata the retina was most heavily populated with nestin positive cells (Fig 2A, B). The increase in the number of nestin positive cells was accompanied by a marked increase in filamentous cell staining. Filamentous cells were superficial (arranged in the ILM and NFL) or radial. Their processes were continuous in places and often contributed to the most superficial element of the ILM, where we also observed less frequently somata (Fig 2C, D). Cells that spanned the retina were similar in morphology to Müller cells (Fig 2). Occasional cells are found with rounded morphology and speckled cytoplasmic staining (Fig 2E), similar to those seen in the posterior retina around the nuclei of neurons. Some cells show both patterns of staining (Fig 2F). Nestin positive cells were occasionally also observed around blood vessels.
Epiretinal membranes
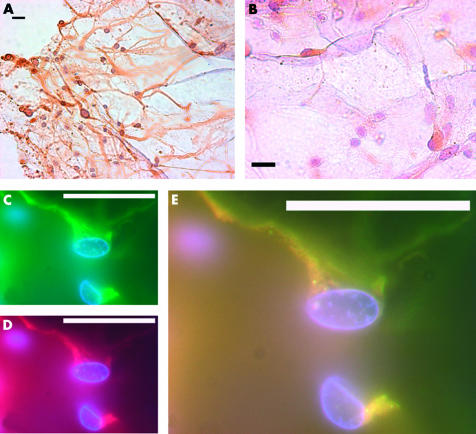
Ectopic nestin positive cells found in clumps (Fig 3A, B) constituted the major cell type in epiretinal membranes (ERM). The nestin positive cells in ERM were elongated and none was speckled. The presence of nestin positive cells in epiretinal membranes is perhaps not surprising as these cells often lie at the innermost aspect of the retina (see above). However the epiretinal membranes were removed from posterior and mid-peripheral retina, where such cells occur in very small numbers or are completely absent. The nestin positive cells in epiretinal membranes were all GFAP positive when these cells were co-stained for GFAP (Fig 3C, D, E).
Figure 3.
Nestin positive cells in epiretinal membranes removed surgically, from the posterior retina. Composite of nestin positive cells in epiretinal membranes removed surgically. All scale bars represent 20 μm. (A) A clump of nestin positive cells, with elongated fibrous processes in an epiretinal membrane removed surgically. All nestin positive cells identified in epiretinal membranes (ERM) had elongated and fibrous processes. (B) A high power view of nestin positive cells in an ERM. Not all cells are nestin positive. Immunofluorescence demonstrates that GFAP (C, green) and nestin (D, red) co-localises in many cells in epiretinal membranes (E).
DISCUSSION
In adult human retina and ERM (surface scarring) removed during retinal surgery, we found different nestin staining patterns associated with cells of neural and glial morphology. The cells with speckled cytoplasm were found in layers where neuronal somata are present and the fibrillary nestin staining cells are morphologically and geographically more similar to glial cells. In ERM these cells co-expressed with GFAP. Different patterns of cytoplasmic staining are not unusual for intermediate filaments in different contexts.12,13 Nestin expression is differently modulated during neuronal and glial differentiation in human neural progenitor cells.14–16 Nestin positive neural progenitor cells have been described in the eyes of adult mammals.4,7,8 Nestin mRNA and protein levels correlate during CNS development2 and reflect the ability of cells to proliferate1 in the brain. Although not all neural progenitors express nestin,1,17 nestin immunoreactivity suggests the presence of neural progenitor cells or cells derived from them. Some post-mitotic cells of the CNS express nestin,1,18 as do some cells of non-CNS origin (including skeletal muscle and peripheral nervous system).2,3,19 In adult human retina, the nestin staining we describe suggests that the ora serrata may be a growth or germinal zone, equivalent to the anatomically similar ciliary margin zone (CMZ). The CMZ is a proliferative area that has been identified in numerous species, including fish, reptiles, birds, marsupials, and mammals, and gives rise to new retinal cells.20
Nestin expression was originally shown by immunocytochemistry to occur in radial glial cells and CNS progenitor cells of the early spinal cord.3 In the developing neural tube proliferating CNS progenitors arise from specific zones.2,3,19,21–23 As the progeny of these cells migrate away they follow radial glial cells,24,25 another mitotically active cell of the progenitor group.21,26 Radial glia are a transient cell population in many parts of the CNS.1,3 Radial glia in the adult mammalian cerebellum retain the capacity to increase their nestin immunoreactivity when they direct the migration of immature cells.27 The expression of nestin in radial cells in adult retina in regions close to the ora serrata suggests that these cells may represent a subset of neural progenitors, able to direct new cells. Without further isolation, temporal, and functional studies we cannot confirm that the nestin positive cells in adult human retina or from retinal scar tissue are neural progenitors.
Nestin positive cells in the adult human retina display morphological and geographical similarity to neurons, including retinal ganglion cells, other neurons, and Müller cells. In the adult human brain nestin positive neurons are found in the hippocampus18 in cells prone to degeneration in Alzheimer’s disease; nestin immunoreactivity in retinal ganglion cells (prone to degeneration in glaucoma) may be comparable.
Neuronal and glial replacement are hypothetical as we do not know if this process is occurring. Nestin positive cells may play a part in the maintenance of the normal retina. Alternatively these cells may upregulate nestin expression in response to an unknown stimulus (for example, cytoskeletal repair). The latter may seem the more feasible in normal retina but does not so readily explain the observations in epiretinal membranes. The presence of progenitor cells explains the varied cellular elements (of uncertain origin28) found in these abnormal scars arising from diverse pathological processes.28 Their presence in ERM implicates them in retinal responses to injury and disease, akin to astroglial scar formation following cortical injury in adult mice.29 The pattern of division and differentiation of progenitors reflects the variety of growth factors and cell signals documented in epiretinal membranes.30–34
The presence of these cells challenges the dogma that the adult human retina does not regenerate or undergo cellular replacement throughout life, possibly similar to the CMZ in other species. Their large numbers in ERM implicates them in retinal responses to disease and injury. Progenitor cell derived elements may be responsible for retinal scars occurring in retinal detachment, inflammation, vascular occlusion, degeneration, idiopathic epiretinal membranes, and macular holes. Retinal disease could divert progenitor cells from homeostasis into scar formation, not to mention increase their division and/or recruitment.
Acknowledgments
We knowledge the technical support of Dr L Xue with the immunocytochemistry of epiretinal membranes.
We are grateful to the National Eye Research Council for their financial support for this work through a grant made to E Mayer in 2000, to support this project, and to the Guide Dogs for the Blind Association. Ed Hughes is supported by an Iris Fund Clinical Research Fellowship and Debra Carter is supported by the Guide Dogs for the Blind Association.
We would like to thank the patients who were all so eager to support this work, by donation of their epiretinal membranes. We also acknowledge the South West Region ethics research committee for their helpful suggestions.
REFERENCES
- 1.Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with central nervous system progenitor cell state in many but not all regions of the developing central nervous system. Dev Brain Res 1995;84:109–29. [DOI] [PubMed] [Google Scholar]
- 2.Lendahl U, Zimmerman LB, McKay R. CNS stem cells express a new class of intermediate filament protein. Cell 1990;60:585–95. [DOI] [PubMed] [Google Scholar]
- 3.Hockfield S, McKay R. Identification of major cell classes in the developing mammalian nervous system. J Neurosci 1985;5:3310–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tropepe V, Coles BL, Chiasson BJ, et al. Retinal stem cells in the adult mammalian eye. Science 2000;287:2032–6. [DOI] [PubMed] [Google Scholar]
- 5.Kirsch M, Schulz-Key S, Wiese A, et al. Ciliary neurotrophic factor blocks rod photoreceptor differentiation from postmitotic precursor cells in vitro. Cell Tissue Res 1998;291:207–16. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun 2000;270:517–21. [DOI] [PubMed] [Google Scholar]
- 7.Sheedlo HJ, Turner JE. Influence of a retinal pigment epithelial cell factor(s) on rat retinal progenitor cells. Brain Res Dev Brain Res 1996;93:88–99. [DOI] [PubMed] [Google Scholar]
- 8.Sheedlo HJ, Brun-Zinkernagel AM, Oakford LX, et al. Rat retinal progenitor cells and a retinal pigment epithelial factor. Brain Res Dev Brain Res 2001;127:185–7. [DOI] [PubMed] [Google Scholar]
- 9.Chacko DM, Rogers JA, Turner JE, et al. Survival and differentiation of cultured retinal progenitors transplanted in the subretinal space of the rat. Biochem Biophys Res Commun 2000;268:842–6. [DOI] [PubMed] [Google Scholar]
- 10.Schraermeyer U, Thumann G, Luther T, et al. Subretinally transplanted embryonic stem cells rescue photoreceptor cells from degeneration in the RCS rats. Cell Transplant 2001;10:673–80. [PubMed] [Google Scholar]
- 11.Palmer TD, Schwartz PH, Taupin P, et al. Progenitor cells from human brain after death. Nature 2001;411:42–3. [DOI] [PubMed] [Google Scholar]
- 12.Prahlad V, Yoon M, Moir RD, et al. Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J Cell Biol 1998;143:159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol 2000;12:79–90. [DOI] [PubMed] [Google Scholar]
- 14.Messam CA, Hou J, Major EO. Coexpression of nestin in neural and glial cells in the developing human CNS defined by a human-specific anti-nestin antibody. Exp Neurol 2000;161:585–96. [DOI] [PubMed] [Google Scholar]
- 15.Messam CA, Hou J, Berman JW, et al. Analysis of the temporal expression of nestin in humna fetal brain derived neuronal and glial progenitor cells. Dev Brain Res 2002;134:87–92. [DOI] [PubMed] [Google Scholar]
- 16.Lothian C, Prakash N, Lendahl U, et al. Identification of both general and region specific embryonic CNS enhancer elements in the nestin promoter. Exp Cell Res 1999;248:509–19. [DOI] [PubMed] [Google Scholar]
- 17.Kukekov VG, Laywell ED, Thomas LB, et al. A nestin negative precursor cell from the adult mouse brain gives rise to neurones and glia. Glia 1997;21:399–407. [DOI] [PubMed] [Google Scholar]
- 18.Gu H, Wang S, Messam CA, et al. Distribution of nestin immunoreactivity in the normal adult human forebrain. Brain Res 2002;943:174–80. [DOI] [PubMed] [Google Scholar]
- 19.Steinert PM, Chou Y-H, Prahlad V, et al. A high molceular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type IV intermediate filament protein. J Biol Chem 1999;274:9881–90. [DOI] [PubMed] [Google Scholar]
- 20.Kubota R, Hokoc JN, Moshiri A, et al. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Dev Brain Res 2002;134:31–41. [DOI] [PubMed] [Google Scholar]
- 21.McKay R. The origins of cellular diversity in the mammalian central nervous system. Cell 1989;58:815–21. [DOI] [PubMed] [Google Scholar]
- 22.Sauer FC. Mitosis in the neural tube. J Comp Neurol 1935;62:377–405. [Google Scholar]
- 23.Hinds JW, Ruffett TL. Cell proliferation in the neural tube: an electron mikcroscopic and Golgi analysis in the mouse cerbral vessicle. Z Zellforsch Mikrosk Anat 1971;115:226–64. [DOI] [PubMed] [Google Scholar]
- 24.Rakic P. Mode of cell migration to the superficial layers of monkey neocortex. J Comp Neurol 1972;145:61–84. [DOI] [PubMed] [Google Scholar]
- 25.Rakic P. Specification of cerebral cortical areas. Science 1988:170–6. [DOI] [PubMed]
- 26.Misson J-P, Edwards MA, Yamamoto M, et al. Mitotic cycling of radial glial cells of the fetal murine cerebral wall: a combined autoradiographic and immunohistochemical study. Dev Brain Res 1988;38:183–90. [DOI] [PubMed] [Google Scholar]
- 27.Sotelo C, Alvarado-Mallart R-M, Frain M, et al. Molecular plasticity of adult Bergmann glial fibres is associated with radial migration of grafted Purkinje cells. J Neurosci 1994;14:124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinores SA, Campochiaro PA, Conway BP. Ultrastructural and electron-immunocytochemical characterisation of cells in epiretinal membranes. Invest Ophthalmol Vis Sci 1990;31:14–28. [PubMed] [Google Scholar]
- 29.Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res 2001;66:317–26. [DOI] [PubMed] [Google Scholar]
- 30.Knott RM, Pascal MM, Ferguson C, et al. Regulation of transforming growth factor-beta, basic fibroblast growth factor, and vascular endothelial cell growth factor mRNA in peripheral blood leukocytes in patients with diabetic retinopathy. Metabolism 1999;48:1172–8. [DOI] [PubMed] [Google Scholar]
- 31.Humphrey MF, Chu Y, Mann K, et al. Retinal GFAP and bFGF expression after multiple argon laser photocoagulation injuries assessed by both immunoreactivity and mRNA levels. Exp Eye Res 1997;64:361–9. [DOI] [PubMed] [Google Scholar]
- 32.Boulton M, Gregor Z, McLeod D, et al. Intravitreal growth factors in proliferative diabetic retinopathy: correlation with neovascular activity and glycaemic management. Br J Ophthalmol 1997;81:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limb GA, Alam A, Earley O, et al. Distribution of cytokine proteins within epiretinal membranes in proliferative vitreoretinopathy. Curr Eye Res 1994;13:791–8. [DOI] [PubMed] [Google Scholar]
- 34.Harada T, Harada C, Mitamura Y, et al. Neurotrophic factor receptors in epiretinal membranes after human diabetic retinopathy. Diabetes Care 2002;25:1060–5. [DOI] [PubMed] [Google Scholar]