Abstract
Background/aims: There is no effective treatment for ischaemic central retinal vein occlusion (CRVO). The two major negative outcomes are neovascular glaucoma (NVG) and severe central visual loss. In this study pars plana vitrectomy (PPV), mild panretinal photocoagulation, and intraocular gas injection were employed to prevent NVG. The potential role of incision of the lamina cribrosa (radial neurotomy) for visual recovery was examined.
Methods: Eight eyes of seven patients with ischaemic CRVO had PPV, mild panretinal photocoagulation, and intraocular perfluoropropane gas injection. Four eyes had radial neurotomies performed. The patients were examined by fundus photography, fundus fluorescein angiography, optical coherence tomography, and Goldmann visual field analysis.
Results: No patients suffered from neovascular glaucoma. Visual recovery was seen in patients with and without neurotomy but some patients had cataract extraction to allow visualisation for PPV. Fundus photography demonstrated reduced engorgement of retinal veins in two of the patients with neurotomy and one with PPV alone. Optical coherence tomography demonstrated macular oedema in three patients with neurotomy and all patients with PPV alone. Segmental visual field loss was seen in one patient with neurotomy suggesting damage to the optic nerve head.
Conclusions: PPV is safe in ischaemic CRVO. Combined with mild PRP and intraocular gas injection the risk of neovascular glaucoma is low. Neurotomy can be added to try to improve the chances of recovery of central vision but may cause additional peripheral visual field loss.
Keywords: optical coherence tomography, pars plana vitrectomy, retinal vein occlusion
Central retinal vein occlusion (CRVO) is the second commonest vascular cause of reduction of vision in the eye. CRVO can be associated with severe irreversible visual loss with improvement of vision in only 20%.1 As yet there is no established treatment. Chorioretinal anastomosis using laser has been successful in improving vision in the non-ischaemic variant of the disorder in selected patients but has been associated with frequent complications.2 This therapy is not used in ischaemic CRVO because of a high complication rate. Recently Opremcak et al have described an operation involving pars plana vitrectomy (PPV) and incision of the optic nerve on the nasal side (neurotomy) in 11 patients and shown an improvement in vision in eight eyes with an average gain of five lines of Snellen acuity.3 It is thought that the neurotomy helps to improve blood flow in the central retinal vein by relieving pressure on the vein as it exits the lamina cribrosa. Although their study showed an improvement in vision there was no assessment of the neurotomy on visual field loss or the effects of PPV alone on CRVO. In addition, they mention that some of their patients had vitreous haemorrhage but fail to indicate how many and what effect this has had on visual recovery.
Iris neovascularisation occurs in 45–80% of patients with ischaemic CRVO.4 Although neurotomy was associated with visual improvement, neovascular glaucoma (NVG) occurred in two patients in Opremcak et al’s study. Theoretically, PPV and intraocular gas injection may increase oxygenation to the retina. However, removal of the vitreous may allow angiogenic factors into the anterior chamber increasing the risk of NVG; therefore, panretinal photocoagulation may be required.
We performed a pilot study to assess the safety of PPV alone or PPV combined with neurotomy in ischaemic CRVO. Visual fields were recorded to determine the effect of the neurotomy on the optic nerve head. In addition we applied mild panretinal photocoagulation (PRP) and injected a long acting gas bubble (perfluoropropane) to try to counteract retinal ischaemia and prevent neovascular glaucoma.
METHODS
Patient selection
Eight eyes of seven consecutive patients with ischaemic CRVO were enrolled. The duration of CRVO was 6 months or less. Relative afferent pupillary defect (RAPD), best corrected Snellen visual acuity (VA), general ophthalmological examination, and fundus photography (FFA) were performed. Ischaemia was defined by the criteria we have previously published.4 Patients were fully informed and gave consent in accordance with the Helsinki declaration. Postoperatively the investigations were repeated in addition to Goldmann visual field analysis and optical coherence tomography (OCT).
Surgical procedure
All eyes had the following surgical procedures:
A three port pars plana vitrectomy
Mild PRP (500–991 coagulates with 0.2 second duration 200 mW in a scatter pattern in the peripheral retina avoiding retinal haemorrhages)
Fluid-gas exchange
12% perfluoropropane in air injected into the vitreous cavity.
Eyes 1–4 and 7 had preoperative posterior vitreous detachments. The posterior hyaloid membrane was detached by active aspiration from the surface of the retina was in eyes 5 and 6. Two patients (6 and 7) required phacoemulsification extraction of a cataractous lens and insertion of an intraocular lens implantation to allow visualisation of the retina for PPV.
Four eyes of three patients had the additional procedure of a radial optic neurotomy in accordance with the description by Opremcak et al.3 Briefly, a microvitreoretinal blade (MVR) was inserted into the nasal disc margin for a depth of approximately 2.5 mm. If bleeding occurred the infusion bottle was raised to stop the haemorrhage.
Assessment of fundus photographs
Three experienced retinal specialists were asked to judge whether there was a difference in preoperative (day before surgery) and postoperative fundus photographs (examination after disappearance of the perfluoropropane gas, range 2–4 months after surgery) for the features of retinal haemorrhaging and venous engorgement. The observers were masked to the identity of the patients. A pair of images from each eye was shown together without the observer knowing which image was preoperative or which postoperative. The observer was asked to choose the image with less haemorrhaging and the image with less venous dilation or to define the images as no difference. The optic discs were obscured to prevent the identification of neurotomy scars. Agreement was required between at least two observers to classify an image as having less haemorrhaging or venous engorgement.
RESULTS
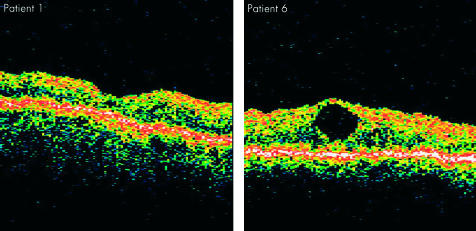
Seven male patients were recruited. The mean age of the patients was 74 years (range 61–88 years) with mean follow up of 10 months (range 5–17 months). Mean duration of symptoms before surgery was 2.9 months (range 1–6 months). Six patients had preoperative relative afferent pupillary defects (RAPDs). Visual acuity results are shown in Table 1. Improvements were seen in both the PPV group alone and the neurotomy group; however, the PPV alone group had the confounding variable of cataract extraction in cases 6 and 7. Three eyes (1, 2, and 4) had iris neovascularisation preoperatively on slit lamp biomicroscopy and gonioscopy. In all eyes this regressed postoperatively. Subjective examination of fundus photography showed a reduction in haemorrhaging in three patients with neurotomy and two patients with PPV alone. A reduction in congestion of the veins was observed in two of the neurotomy group and one of the PPV alone patients as illustrated by the Figures 1 and 2. Visual field loss appropriate to ischaemic CRVO was seen in all cases. However a segmental temporal visual field loss was seen in one of the eyes with neurotomy, patient 1, which was not seen in the PPV alone group (Figs 3 and 4). OCT images showed cystoid macular oedema in three of four eyes of the neurotomy group and all of the eyes with PPV alone (Fig 5). No cases of NVG occurred despite three patients having preoperative iris neovascularisation.
Table 1.
Results of surgery and investigations
| Visual acuity | |||||||||
| Case | Age (years) | Neurotomy | Venous dilatation | Haemorrhages | BVNS | OCT | Visual fields | Preop | Postop |
| 1 | 61 | Yes | No change | Less postop | Yes | Normal | Supero-temporal defect | CF | CF |
| 2 | 80 | Yes | Less postop | Less postop | No | CMO + SRF | Large blind spot | HM | CF |
| 3 R | 82 | Yes | No change | More postop | Yes | CMO + SRF | Central defect | CF | 6/60 |
| 3 L | 82 | Yes | Less postop | Less postop | Yes | Mild CMO | Severe constriction | CF | 6/60 |
| 4 | 88 | No | No change | Less postop | No | SRF | Severe constriction | CF | CF |
| 5 | 63 | No | No change | No change | No | Foveal cyst | Full | CF | 6/36 |
| 6 | 72 | No | No change | No change | No | Foveal cyst | Full | 6/36 | 6/12 |
| 7 | 70 | No | Less postop | Less postop | No | No foveal dip | Central defect | HM | CF |
Preop = preoperative, postop = postoperative, BVNS = blood vessels in the neurotomy site, OCT = optical coherence tomography, CMO = cystoid macular oedema, SRF = subretinal fluid, HM = hand movements, CF = counting fingers.
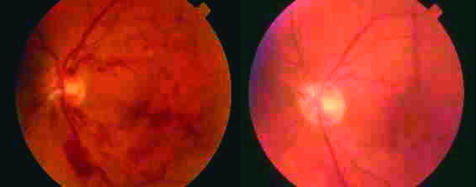
Figure 1.
Colour fundus photographs of patient 3 are shown with neurotomy preoperative image on the left and postoperative on the right showing loss of haemorrhaging and narrowing of venous calibre.
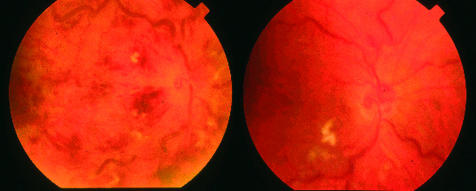
Figure 2.
Colour fundus photographs of patient 7 are shown with PPV alone, preoperative image on the left and postoperative on the right showing loss of haemorrhaging but persistent venous dilation.
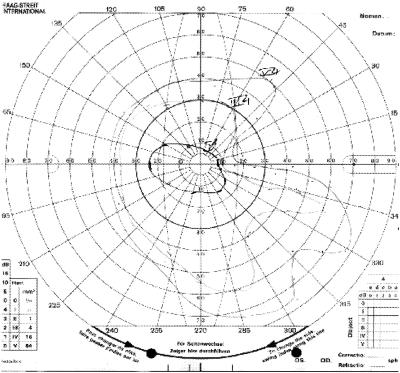
Figure 3.
Visual fields of patient 1 with neurotomy with sectoral visual field loss from the incision.
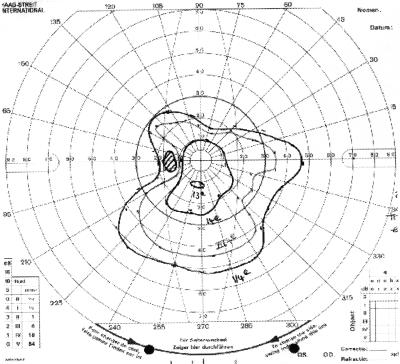
Figure 4.
Visual field of patient 4 with PPV only showing severe constriction from the CRVO.
Figure 5.
OCTs of patient 1 treated with neurotomy with no CMO but poor visual recovery and patient 6 with PPV alone with a foveal cyst but with 6/12 vision.
DISCUSSION
The pathogenesis of CRVO is poorly understood.5,6 Pathological evidence suggests the site of obstruction is situated at the lamina cribrosa, although histological samples of early CRVO are rare.7 The anatomy of the normal central retinal vein appears to show a constriction of the vein as it passes through the lamina cribrosa.8 This may predispose the vein to occlusion, thereby reducing its retinal blood flow.9 Secondary ischaemia of the retina occurs from the stasis of blood flow in the capillaries caused by back pressure from the occluded venous system.
PPV may have beneficial effects upon retinal ischaemia by allowing circulation in the vitreous cavity of fluid oxygenated by unaffected retina or other sites in the eye such as the ciliary body. This may partly explain the advantageous effects of early vitrectomy on diabetic retinopathy. Using the principles of Fick’s diffusion equation we speculate that the insertion of a high molecular weight gas (in this case perfluoropropane) into the vitreous cavity might also improve oxygenation. At least 95% of ocular blood flow passes through ciliary circulation primarily the choroid. This can be regarded as a source of oxygen divided from the vitreous cavity by a semipermeable membrane—that is, the retina. The perfluoropropane gas draws oxygen and other small molecular weight gases through the retina into the vitreous cavity in an active equilibrium. Hopefully the oxygen can be utilised by the ischaemic retinal tissues. Gradually the perfluoropropane gas is lost over a period of 2 months. Theoretically, this may reduce neovascular complications over the crucial early period of the CRVO natural history.10 For example, many CRVO patients gradually develop capillary non-perfusion up to 3 months after onset with iris neovascularisation usually occurring in the first 6 months.
There was a risk that PPV would allow release of angiogenic factors into the anterior chamber causing severe NVG. For this reason in this pilot study we also performed mild PRP. The number of burns given was much less than the dosage applied in the CRVO study. This strategy was successful in reversing the neovascularisation in three patients and no cases of NVG were seen in comparison to Opremcak et al who encountered two patients with NVG and poor visual outcome.
The visual acuity results are hard to interpret because of the need to perform cataract extractions in two of the patients with PPV in order to visualise the retina for surgery. Excluding these two patients, small improvements in vision were observed in four of the remaining six eyes. Without a control group it is impossible to determine whether this is better than expected from the natural history of the disorder. The OCT scans illustrate that there is persistent disruption to the anatomy of the macula postoperatively. Possibly the damage from the ischaemic process in CRVO is too severe to reverse.
The mechanism of action of the neurotomy is uncertain. The intention is to decompress the central retinal vein in the nerve. However, three of the eyes with neurotomy had evidence of the formation of blood vessels in the neurotomy site. Perhaps these represent a chorioretinal anastomosis aiding venous drainage from the retina. However, investigators will need to examine the effect of PPV in these patients before they attribute their changes to the neurotomy. Results in this study were inconclusive with two patients who received radial neurotomy and one with PPV alone showing a reduction in the congestion in the retinal veins on fundus photography in the postoperative period. Also, the results of OCT were only slightly more favourable in the neurotomy group.
Incising the optic nerve to create the neurotomy has risks. All four eyes of the patients suffered a peroperative bleed from the incision which was easily controlled by elevation of the intraocular pressure but which may indicate damage to the vasculature around the optic nerve.11 One patient in our study had a specific focal visual field defect appropriate to the site of neurotomy indicating damage to the nerve fibres or to the blood supply of the nerve head, whereas the patients with PPV showed only constriction of the visual field or central defects as expected in ischaemic CRVO. The risks, however, are outweighed if PPV and neurotomy prove to be a reliable method of restoring central vision in CRVO.
In conclusion, PPV is safe in CRVO. Combined with mild PRP and intraocular gas injection the risk of neovascular glaucoma is low. Neurotomy can be added to try to improve the chances of recovery of central vision but carries a risk of visual field loss.
Supplementary Material
REFERENCES
- 1.The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol 1997;115:486–91. [DOI] [PubMed] [Google Scholar]
- 2.McAllister IL, Douglas JP, Constable IJ, et al. Laser-induced chorioretinal venous anastomosis for nonischemic central retinal vein occlusion: evaluation of the complications and their risk factors. Am J Ophthalmol 1998;126:219–29. [DOI] [PubMed] [Google Scholar]
- 3.Opremcak EM, Bruce RA, Lomeo MD, et al. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina 2001;21:408–15. [DOI] [PubMed] [Google Scholar]
- 4.Williamson TH. Central retinal vein occlusion: what’s the story? Br J Ophthalmol 1997;81:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Heuven WA, Hayreh MS, Hayreh SS. Pathogenesis of ‘central retinal vein occlusion’. Bibl Anat 1977:1–5. [PubMed]
- 6.Hayreh SS, van Heuven WA, Hayreh MS. Experimental retinal vascular occlusion. I. Pathogenesis of central retinal vein occlusion. Arch Ophthalmol 1978;96:311–23. [DOI] [PubMed] [Google Scholar]
- 7.Green WR, Chan CC, Hutchins GM, et al. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Trans Am Ophthalmol Soc 1981;79:371–422. [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor AW, Sehu W, Williamson TH, et al. Morphometric assessment of the central retinal artery and vein in the optic nerve head. Can J Ophthalmol 1993;28:320–4. [PubMed] [Google Scholar]
- 9.Williamson TH, Baxter GM. Central retinal vein occlusion, an investigation by color Doppler imaging. Blood velocity characteristics and prediction of iris neovascularization. Ophthalmology 1994;101:1362–72. [DOI] [PubMed] [Google Scholar]
- 10.Central Vein Occlusion Study Group. Central vein occlusion study of photocoagulation therapy. Baseline findings. Online.J Curr Clin Trials 1993;Doc No 95:6021. [PubMed] [Google Scholar]
- 11.Gauntt CD, Williamson TH, Sanders MD. Relationship of the distal optic nerve sheath to the circle of Zinn. Graefes Arch Clin Exp Ophthalmol 1999;237:642–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.