Abstract
Aim: To evaluate the efficacy of latanoprost (Xalatan) as adjunctive therapy in port wine stain related paediatric glaucoma.
Methods: A retrospective non-randomised study. Patients with previous surgical intervention and medical treatment were included. Measurements were recorded from clinic and/or examination under anaesthetic (EUA) visits. A successful outcome was considered to be patients who required no further intervention following initiation of latanoprost, with stable glaucoma factors as well as drop in intraocular pressure.
Results: 14 patients and 17 eyes were reviewed in total. The mean age of glaucoma diagnosis was 2.59 years (0.1–5.25 years) and of commencing latanoprost was 6.8 years (1.40–12.90 years). Percentage success at 1 month, 3 months, 6 months, and 1 year was 70.6%, 64.7%, 58.9%, and 47.1%, respectively, of eyes treated which translated to 71.4%, 64.2%, 57.1%, and 50% respectively of patients treated.
Conclusions: A trial of latanoprost as adjunctive therapy in patients with port wine stain related glaucoma may temporise the need for surgery; with 50% of patients being controlled at 1 year follow up. Lack of efficacy was detected as early as 1 month following commencement of treatment.
Keywords: latanoprost, port wine stain, paediatric ophthalmology
Port wine stain (naevus flammeus) may be isolated or associated with certain syndromes such as Sturge-Weber syndrome (SWS) or cutis mamorata telengiectasia congenita (CMTC). The incidence of glaucoma in patients with SWS has been reported between 30%1,2 and 71%,3 with those who have episcleral involvement invariably having raised intraocular pressures.4 The mechanisms involved are controversial but are thought to include anomalous angles in younger age groups and increased episcleral outflow resistance.4
Latanoprost (Xalatan, Pharmacia and Upjohn), a prostaglandin F2a analogue, lowers intraocular pressure (IOP) by increasing uveoscleral outflow. It has been shown to be efficacious when used as adjunctive therapy5 and as monotherapy6 in adult glaucoma. The efficacy and safety of latanoprost is not well known in children.7,8 We report our experience of latanoprost in a group with port wine stain related glaucoma.
PATIENTS AND METHODS
A database of patients receiving latanoprost treatment at Great Ormond Street Hospital for Children was reviewed and those with port wine stain related glaucoma were identified.
The clinical records of all patients were reviewed retrospectively. Data collected included age at presentation and onset of glaucoma, pre-latanoprost medications and surgical interventions, the pre-latanoprost and post-latanoprost IOP, cycloplegic refraction, horizontal corneal diameter and cup-disc ratio. IOP measurements were obtained by a variety of methods (Goldmann tonometry, Tonopen, Perkins tonometry, Keeler Pulsair 3000) depending on the child’s cooperation. IOP was measured while the child was awake if possible. When examination under anaesthesia was required the anaesthetic gas inhalant Sevoflurane was used and measurements of IOPs were made with the Perkins applanation tonometer immediately after induction and before intubation. Sequential reliable visual fields were not available in any patient.
Success was defined as the stabilisation of ocular parameters without the need for further surgical or medical intervention. Success was based not only on IOP recordings but also on other ocular parameters such as improved corneal clarity, stable corneal diameter, stable cup-disc ratios, and refractive status (that is, lack of myopic shift).
Those who failed the trial of latanoprost were compared to those who succeeded to try identify any predictive risk factors. Variables considered included age at diagnosis of glaucoma, age at commencing latanoprost, previous surgical interventions, previous medication and pre-latanoprost corneal diameter. Any side effects causing cessation of latanoprost therapy were also noted.
RESULTS
Fourteen patients (17 eyes) were reviewed. Nine had isolated port wine stain related glaucoma, one had CMTC, and four had SWS. Three patients had bilateral port wine stain. No patient suffered side effects on commencing latanoprost, which necessitated its cessation.
Patient characteristics are shown in Table 1. The mean age of glaucoma diagnosis was 2.59 years and the mean age of commencing latanoprost was 6.8 years. All patients were on other antiglaucoma medication before commencing latanoprost. The mean number of pre-latanoprost medication was 1.92 (range 1–3) and included betaxolol, pilocarpine, and brimonidine (one patient had used propine previously). The mean number of surgical interventions pre-latanoprost was 1.15 (range 0–6) and included goniotomy, trabeculectomy, and cycloablation.
Table 1.
Patient characteristics
| Mean | Range | SD | |
| Age at glaucoma diagnosis | 2.596 | 0.1–5.25 | 2.673 |
| Age at commencing latanoprost | 6.80 | 1.40–12.90 | 3.299 |
| Medications pre-latanoprost | 1.92 | 1–3 | 0.760 |
| Surgical intervention pre-latanoprost | 1.154 | 0–6 | 1.725 |
| IOP pre-latanoprost | 24.92 | 14–35 | 5.894 |
| Cup-disc ratio pre-latanoprost | 0.60 | 0.2–0.9 | 0.197 |
Pre-latanoprost variables previously mentioned did not show a statistically significant difference between success and failure groups which is probably because of a small population number (Table 2). Those who failed, however, were noted to have higher IOPs, required more topical medication and previous intervention, and larger corneal diameters.
Table 2.
Pre-latanoprost ocular variables (SD) of patients who succeeded/failed at 12 months
| Success | Failure | p Value | |
| Age at diagnosis of glaucoma (years) | 2.7 (3.6) | 2.5 (2.2) | 0.87 |
| Age at commencing latanoprost (years) | 8.2 (4.2) | 5.9 (2.5) | 0.24 |
| Number of previous surgery | 0.4 (0.5) | 1.6 (2.1) | 0.22 |
| Number of topical medications | 1.6 (0.9) | 2.1 (0.6) | 0.24 |
| Intraocular pressure (mm Hg) | 21.2 (4.0) | 27.3 (5.8) | 0.07 |
| Cup-disc ratio | 0.5 (0.2) | 0.6 (0.2) | 0.33 |
| Corneal diameter (mm) | 12.2 (0.8) | 13.1 (0.8) | 0.06 |
The percentage of eyes in the success group at 1 month, 3 months, 6 months, and 1 year were: 70.6%, 64.7%, 58.9%, and 47.1%, respectively; this translated to 71.4%, 64.2%, 57.1%, and 50%, respectively, of patients treated.
DISCUSSION
As far as we are aware, this is the largest study of its kind currently reported from a single centre.3,9–12 This is significant because many variables exist in the management of paediatric glaucomas such as composition of the treatment regimen and the temporal progression along this treatment regimen. At our centre all management is coordinated by one of the authors (KKN) for patients with port wine stain related paediatric glaucoma. As such the composition of, and progression along, the treatment regimen are relatively constant between patients, which allows a better analysis of the effect of one component of the treatment regimen—latanoprost in this particular case. This is less likely to be the case between different centres unless a prospective study with uniform treatment regimens is used, which is not the case for latanoprost.3,9–12
The management of port wine stain related glaucoma is difficult and controversial.13,14,15,16 These patients often exhibit lower rates of success compared with patients who have primary congenital glaucoma.17
Medical treatment is often the first line treatment of choice in port wine stain related glaucomas14,18 and when effective is preferable to surgical intervention.17 Cryocoagulation of the ciliary body combined with topical medication has also been shown to be an effective and safe treatment option in the management of these patients.19 Failure of medical control invariably leads to surgical intervention,10,17,20 which carries a high risk of complications including postoperative hypotony, retinal detachments, and expulsive haemorrhage.10,17,21,22
Outcome measures of early childhood glaucoma are different from those of adult or adolescent patients because the IOP may not be reliable; several factors can influence the IOP reading, because the child is distressed at the time of taking the pressure awake or because the child is under anaesthesia, giving an artefactual result.23–25 Also reliable sequential visual field monitoring is not always possible as in our cases. For this reason the whole picture—that is, corneal clarity, horizontal corneal diameter, cup-disc ratio, refraction and/or axial length must be taken into account. Some parameters, such as corneal diameter, refraction, and cup-disc ratio may remain stable despite an increase in recorded IOP or vice versa. These factors do not appear to have been considered in the previous reports on the use of latanoprost in port wine stain related paediatric glaucoma.
Latanoprost lowers IOP by increasing uveoscleral outflow. It is thought that it reduces flow resistance through the spaces between the bundles of the ciliary muscle by relaxing the muscle fibres26 and by inducing remodelling of the extracellular matrix.27
Theoretically, the raised episcleral venous pressure around these eyes should counter this decrease in outflow and other authors,4,9,17 have suggested this is why latanoprost did not appear to be as useful in their patients. It is possible, however, that the dynamics of flow can be more complex than this, depending on where the venous malformations actually lie, suggesting that in some patients it might prove useful.
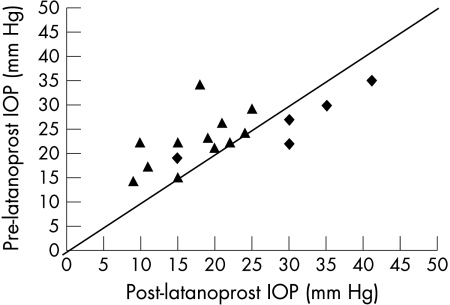
In this and previous studies,4,9,17 there seems to be a subgroup of patients who fail to respond to latanoprost therapy. Younger patients tend to fail because of associated angle anomalies whereas older children tend to do better.3,9,11 This may be because in early onset port wine stain related glaucoma the anterior chamber angle is often similar to that found in congenital glaucomas. Later onset juvenile glaucomas tend to have normal angles.3,11 In our study, five (29.4%) eyes failed to respond within the first month (Fig 1). It is noteworthy that the mean ages at diagnosis in the success and failure group of our study (see Table 2) are approximately equal, suggesting that a younger age at diagnosis alone may not preclude the successful use of latanoprost as adjunctive therapy. Patients who received goniotomy before initiation of latanoprost were also included in the study. This patient group deserves attention because they share the same surgical risk as their counterparts who never had surgery.
Figure 1.
Graph showing intraocular pressure (IOP) 1 month pre-latanoprost and post-latanoprost in the success (triangles) and failure (diamonds) groups. Note that one eye is deemed to be a failure in terms of outcome measure but has a reduced IOP compared to the pre-latanoprost IOP.
Enyedi et al9 reported their experience with latanoprost in a heterogeneous group of children with paediatric glaucoma but within this group they reported complete non-response in the port wine stain related subgroup. Yang et al,11 however, found a failure rate of 66.6% in their patient group at 1 month post-latanoprost. Altuna and colleagues10 from nine centres found a success rate of 16.7% at 6 months. Our success rate for 6 months was 58.9% of treated eyes. This is most probably because our outcome measures for success did not rely on IOP alone, unlike the previous reports.9,10,11
In our study, although there is a suggestion (Table 2) that patients who failed treatment were more likely to have more intractable glaucoma (that is, have higher IOP, require more topical medication and previous intervention, and larger corneal diameters), no statistically significant difference was identified.
It has been suggested that repetitive stretch and straining forces on the trabecular meshwork reduce the function of matrix metalloproteinases (MMP) and alter the extracellular matrix components, causing a further increase in IOP and that this may reduce the effective MMP levels exerted by latanoprost.28–30 Therefore, the presence of buphthalmos itself may reduce the effectiveness of latanoprost. Further studies need to be conducted to confirm this hypothesis.
Almost one third of our patients failed to respond to treatment within a month following initiation of latanoprost. However, 50% of patients (and 47% of eyes) appeared to be effectively controlled at 12 months post-latanoprost.
We therefore suggest a trial of latanoprost as adjunctive therapy in patients with port wine stain related paediatric glaucoma. Patients should be reviewed at least 1 month following initiation of latanoprost therapy so that those who do fail treatment can be identified and alternative intervention initiated promptly.
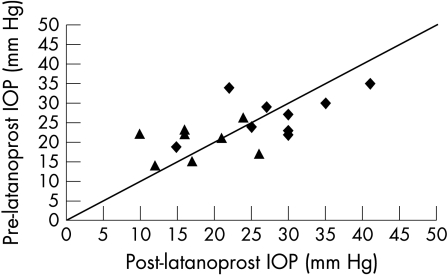
Figure 2.
Graph showing intraocular pressure (IOP) 12 months post-latanoprost compared to pre-latanoprost IOP in the success and failure groups. Note again that the outcome measure of success (triangles) or failure (diamonds) is not dependent on drop in IOP alone.
The authors have no proprietary or financial interest in any of the products mentioned in this manuscript.
REFERENCES
- 1.Tripathi BJ, Tripathi RC, Cibis GW. Sturge-Weber syndrome. Encephalotrigeminal angiomatosis. In: Gold DH, Weingeist TA, eds. The eye in systemic disease. Philadelphia: JB Lippincott, 1990;148:443–7.
- 2.Duke-Elder S, Jay B. Encephalo- (oculo)-facial angiomatosis (the Sturge Weber syndrome). In: Duke-Elder S, ed. System of ophthalmology. Diseases of the lens and vitreous; glaucoma and hypotony. Vol 11. London: Henry Kimpton, 1969:637–40.
- 3.Sullivan TJ, Clarke MP, Morin JD. The ocular manifestations of the Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus 1992;29:349–6. [DOI] [PubMed] [Google Scholar]
- 4.Phelps CD. The pathogenesis of glaucoma in Sturge-Weber syndrome. Ophthalmology 1978;85:276–86. [DOI] [PubMed] [Google Scholar]
- 5.Patelska B, Greenfield DS, Liebmann JM, et al. Latanoprost for uncontrolled glaucoma in a compassionate case protocol. Am J Ophthalmol 1997;124:279–86. [DOI] [PubMed] [Google Scholar]
- 6.Hedman K, Alm A. A pooled-data analysis of three randomized, double-masked, six-month clinical studies comparing the intraocular pressure reducing effect of latanoprost and timolol. Eur J Ophthalmol 2000;10:95–104. [DOI] [PubMed] [Google Scholar]
- 7.Brown SM. Increased iris pigment in a child due to latanoprost. Arch Ophthalmol 1998;116:1683–4 (erratum Arch Ophthalmol 1999;117:751). [PubMed] [Google Scholar]
- 8.Schmidtborn F. Systemic side effects of Latanoprost therapy in children with aniridia and glaucoma. Ophthalmologe 1998;95:633–4. [DOI] [PubMed] [Google Scholar]
- 9.Enyedi LB, Freedman SF, Buckley EG. The effectiveness of latanoprost for the treatment of pediatric glaucoma. J AAPOS 1999;1:33–9. [DOI] [PubMed] [Google Scholar]
- 10.Altuna JC, Greenfield DS, Wand M, et al. Latanoprost in glaucoma associated with Sturge-Weber syndrome: benefits and side effects. J Glaucoma 1999;8:199–203. [PubMed] [Google Scholar]
- 11.Yang CB, Freedman SF, Myers JS, et al. Use of Latanoprost in the treatment of glaucoma associated with Sturge-Weber Syndrome. Am J Ophthalmol 1998;126:600–2. [DOI] [PubMed] [Google Scholar]
- 12.Cibis GW, Tripathi RC, Tripathi BJ. Glaucoma in Sturge-Weber syndrome. Ophthalmology 1984;91:1061–71. [DOI] [PubMed] [Google Scholar]
- 13.Taylor RH, Ainsworth JR, Evans AR, et al. The epidemiology of pediatric glaucoma: the Toronto experience. J AAPOS 1999,3:308–15. [DOI] [PubMed] [Google Scholar]
- 14.Awad AH, Mullaney PB, Al-Mesfer S, et al. Glaucoma in Sturge-Weber syndrome. J AAPOS 1999,3:40–5. [DOI] [PubMed] [Google Scholar]
- 15.Sujansky E. Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol 1995,10:49–58. [DOI] [PubMed] [Google Scholar]
- 16.Morlet N. Goldberg I. Sturge-Weber syndrome and secondary glaucoma. Aust N Z J Ophthalmol 1993,21:271–2. [DOI] [PubMed] [Google Scholar]
- 17.Iwach AG, Hoskins HD, Hetherington J, et al. Analysis of surgical and medical management of glaucoma in Sturge Weber syndrome. Ophthalmology 1990;97:904–9. [DOI] [PubMed] [Google Scholar]
- 18.McMahon CD, Hetherington J, Hoskins HD, et al. Timolol and pediatric glaucomas. Ophthalmology 1981;88:249–52. [DOI] [PubMed] [Google Scholar]
- 19.Van Emelen C, Goethals M, Dralands L, et al. Treatment of glaucoma in children with Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus 2000;37:29–34. [DOI] [PubMed] [Google Scholar]
- 20.Hamush NG, Coleman AL, Wilson MR. Ahmed glaucoma valve implant for management of glaucoma in Sturge-Weber syndrome. Am J Ophthalmol 1999;128:758–60. [DOI] [PubMed] [Google Scholar]
- 21.Christensen GR, Records RE. Glaucoma and expulsive haemorrhage mechanisms in the Sturge Weber syndrome. Ophthalmology 1979;86:1360–6. [DOI] [PubMed] [Google Scholar]
- 22.Mandal AK. Primary combined trabeculotomy-trabeculeculectomy for early-onset glaucoma in Sturge-Weber syndrome. Ophthalmology 1999;106:1621–7. [DOI] [PubMed] [Google Scholar]
- 23.Duman A, Ogun CO, Okesli S. The effect on intraocular pressure of tracheal intubation or laryngeal mask insertion during sevoflurane anaesthesia in children without the use of muscle relaxants. Paediatr Anaesth 2001;11:421–4. [DOI] [PubMed] [Google Scholar]
- 24.Rudolph P. The intraocular pressure during ketamine and halothane anaesthesia Anaesthesist 1974;23:245–8. [PubMed] [Google Scholar]
- 25.Degenne S, Benck P, Gerhard JP, et al. [Alterations in intraocular pressure in children examined by Goldmann’s applanation tonometer under general anesthesia.] Arch Ophtalmol Rev Gen Ophtalmol 1968;l28:359–74. [PubMed] [Google Scholar]
- 26.Poyer JF, Millar C, Kaufman PL. Prostaglandin F2 alpha effects on isolated rhesus monkey ciliary muscle. Invest Ophthalmol Vis Sci 1995;36:2461–5. [PubMed] [Google Scholar]
- 27.Lindsey JD, Kashiwagi K, Kashiwagi F, et al. Prostaglandins alter extracellular matrix adjacent to human ciliary muscle cells in vitro. Invest Ophthalmol Vis Sci 1997;38:2214–23 [PubMed] [Google Scholar]
- 28.Wu Dunn D. The effect of mechanical strain on matrix metalloproteinase production by bovine trabecular meshwork cells. Curr Eye Res 2001;22:394–7. [DOI] [PubMed] [Google Scholar]
- 29.Ocklind A. Effect of latanoprost on the extracellular matrix of the ciliary muscle. A study on cultured cells and tissue sections. Exp Eye Res 1998;67:179–91. [DOI] [PubMed] [Google Scholar]
- 30.Lindsey JD, Kashiwagi K, Kashiwagi F, et al. Prostaglandin action on ciliary smooth muscle extracellular matrix metabolism: implication for uveoscleral outflow. Surv Ophthalmol 1997;41(suppl):S53–9. [DOI] [PubMed] [Google Scholar]