Abstract
Aims: To evaluate umbilical cord serum therapy as a means of promoting the healing of persistent corneal epithelial defects.
Methods: Umbilical cord serum or autologous serum drops were used to promote the healing of persistent epithelial defects. The study design was a prospective randomised controlled clinical trial. 60 eyes of 59 patients were divided into two groups, 31 in the cord serum group and 29 in the autologous serum control group. Epithelial defects measuring at least 2 mm in linear dimension resistant to conventional medical management were included. Serial measurements of the size of the epithelial defects—namely, two maximum linear dimensions perpendicular to each other, and the area and perimeter was done at start of therapy and follow up days 3, 7, 14, 21. Rate of healing of the epithelial defects were measured as percentage decrease from the baseline parameter at each subsequent follow up. The data were analysed by the non-parametric Wilcoxon rank sum test using STATA 7.0.
Results: The median percentage decrease in the size of the epithelial defect was significantly greater in the cord serum group at days 7, 14 and 21 (p<0.05) when measured in terms of the area and perimeter. A greater number of patients showed complete re-epithelialisation with umbilical cord serum (n = 18) than with autologous serum (n = 11) (Pearson χ = 0.19). None of the patients reported any side effects or discomfort with either treatment.
Conclusions: Umbilical cord serum leads to faster healing of the persistent corneal epithelial defects refractory to all medical management compared to autologous serum.
Keywords: autologous serum, cord serum, corneal epithelial defect
The corneal epithelium shows a “wound healing response” in the event of a breach in its integrity.1 However, in certain conditions the defect may not heal.2
The pathogenesis of persistent epithelial defects may often be unclear3 and the various causes as reported in literature are tear film abnormalities, intrinsic epithelial and basement membrane disorders, lid abnormalities, metabolic disturbances, iatrogenic, trauma, chemical burns, immunological disorders, neurogenic, nutritional, infectious, and inflammation.4,5
Fetal bovine serum differentially modulates the clonal growth and differentiation of cultured limbal and corneal epithelium,6 as well as increased migration.7
We hypothesised that umbilical cord blood contains potentially useful growth factors,8,9 and this study aimed to evaluate beneficial role of cord blood serum in persistent corneal epithelial defects.
PATIENTS AND METHODS
Sixty eyes of 59 patients with persistent epithelial defects were enrolled at random from the cornea service at our centre. A persistent epithelial defect was defined as “a corneal epithelial defect persisting for at least 2 weeks with a minimum diameter of 2 mm along the greatest axis without improvement despite conventional treatment such as tear supplements and bandage contact lenses.” Patients of any age group, preferably cooperative for digital photography, were included. Pregnant and lactating women, patients unable to give consent for the therapy, those with active ocular or lid infections, patients with lid abnormalities contributory to the epithelial defect, impending perforations, elevated intraocular pressure, and with severe limbal ischaemia were excluded from the study.
The patients were counselled regarding the nature of the study, the therapy being used and its potential benefits and adverse effects. Written informed consent was given. The study followed the tenets of the Helsinki declaration and was approved by the institutional review board. Patients underwent a thorough examination, which involved a comprehensive ocular and medical history and examination including record of the best corrected visual acuity and slit lamp biomicroscopy. Tear function tests, Schirmer’s (basic) and tear break up time, were performed in all patients in both the involved and the fellow eye. Tactile intraocular pressure was recorded for each patient. A photographic documentation of the epithelial defect was done with and without fluorescein staining. The size of the epithelial defect was measured at its greatest dimension, along two perpendicular axes with a slit lamp micrometer and later also with digital image analysis software Image Pro Plus.10
After examination the patients were first put on a 1 week washout period with normal saline drops and preservative free chloramphenicol drops to clear the epithelium of all toxic preservatives. They were then started on therapy with either umbilical cord serum or autologous serum after measuring the epithelial defect, the day of starting therapy being defined as day 0.
To carry out the randomisation a random number table was used to allocate each eye labelled from 1–60 to either of the groups. Each patient had his blood drawn for preparation of autologous serum, which was prepared according to the standard protocol at the ocular pharmacology department at our centre and returned with serum drops labelled as “serum,” which could be either autologous or cord blood serum. The drops were to be instilled in the affected eye six times a day with no other concurrent lubricant therapy. The allocation of the two groups was divulged after the study was completed, thereby masking both the patients and the investigator from previous knowledge of the groups to which they were assigned.
The umbilical cord blood was collected from mothers with uncomplicated caesarean section deliveries after obtaining informed consent and screening for the parenterally transmitted viral diseases, hepatitis B and C viruses, and HIV, investigations being repeated at the time of delivery if tested previously in the first trimester. The blood was collected by directly cannulating the umbilical vein by the component transfer bag (Terumo Penpol) plastic cannula with all sterile precautions after the baby was removed from the field and the umbilical cord was clamped with a plastic clamp. The blood was allowed to drain by gravity from the placenta. When the blood flow stopped, the needle was removed from the umbilical cord and the blood in the tubing was milked into the bag to maximise collection. No anticoagulants were used during the procedure. The blood was allowed to clot and the serum was centrifuged at 1500 rpm for 5 minutes to separate it completely. It was then diluted using sterile saline solution and dispensed in plastic containers as a 20% solution without any antibiotics. The serum was stored at −4°C in a refrigerator. A culture for bacterial, fungal, and viral contaminants was sent on each occasion when a fresh serum batch was prepared. The patients using the serum were asked to store it in a cool place, preferably in a refrigerator or ice box and look out for thread-like floating objects in the serum. The autologous serum drops were also prepared from the patients’ blood in the same manner.
The patients were followed up on days 3, 7, 14, 21. The variables recorded at each follow up visit were the best corrected visual acuity, the maximum size of the epithelial defect along two perpendicular axes measured with a slit lamp micrometer, and also a digital photograph of the same, a record of the side effects if any, and the digital intraocular pressure. After the 21st day the patients who did not heal were re-evaluated and were advised to have a tarsorrhaphy or an amniotic membrane transplantation.
Statistical analysis
Data were recorded on a predesigned proforma and managed on an Excel spreadsheet. All the entries were checked for any error. Distribution of categorical variables in the two groups was compared using the χ2 test. For quantitative variables following normal distribution we used mean and standard deviation as the summary statistics. For variables following non-normal distribution, we computed median (range) as summary statistics. The various baseline characteristics in the two groups were statistically compared. We found age as the only variable that was not similar in the two groups, therefore, we used repeated measures analysis to find out if age was a confounder. Student’s t test was used to compare mean values in the two groups. Median values in the two treatment groups were compared using Wilcoxon rank sum test. STATA 7.0 statistical software was used for data analysis. In this study, a p value less than 0.05 has been considered as statistically significant.
RESULTS
Of 60 eyes of 59 patients enrolled, 29 were randomised to the autologous serum group and 31 to the cord serum group. One patient had both the eyes affected. The demographic profile and primary diagnoses of the patients is presented in Tables 1 and 2.
Table 1.
Demographic profile of the patients in the two groups
| Variables | Autologous serum group | Umbilical cord serum group |
| Number of patients | 29 | 31 |
| Mean age (SD) (years)* | 47.8 (19.8) | 33.8 (16.8) |
| Sex distribution | M = 23 (80.6%) | M = 22 (72.4%) |
| F = 6 (19.3%) | F = 9 (27.6)% |
*For age distribution p = 0.004.
Table 2.
Primary diagnoses of the patients in the two groups
| Primary diagnosis | Autologous serum (n = 29) | Umbilical cord serum (n = 31) |
| No (%) | No (%) | |
| Post penetrating keratoplasty | 18 (62.87) | 9 (29.03) |
| Chemical injury | 3 (10.34) | 7 (22.58) |
| Post vitreoretinal surgery | 2 (6.9) | 3 (9.68) |
| Post keratitis | 2 (6.9) | 4 (12.9) |
| Neurotrophic ulcer | 4 (13.79) | 8 (25.81) |
*All the variables were statistically similar in the two groups (p>0.05).
At initial presentation most of the patients (78%) had a visual acuity of <3/60. The distribution of visual acuities between the two groups was not significantly different.
A majority of the patients (63.3%, n = 38) had a Schirmer test of more than 10 mm in the first 5 minutes, 25% (n = 15) had a Schirmer test ranging between 5 mm and 10 mm, and the remaining 11.7% (n = 7) had a Schirmer test of less than 5 mm.
Of the 60 patients 48.3% (n = 29) healed on either therapy; 58.06% (n = 18/31) in the cord serum group and 37.93% (n = 11/29) in the autologous serum group (Pearson χ2 = 0.19). The total number of patients showing re-epithelialisation in the different groups is shown in Table 3. There was no significant gain in visual acuity in both the groups in cases showing complete re-epithelialisation.
Table 3.
Complete re-epithelialisation in individual groups of patients
| Primary diagnosis | Autologous serum (n = 29) | Umbilical cord serum (n = 31) | ||
| Healed (n = 11) | Not healed (n = 18) | Healed (n = 18) | Not healed (n = 13) | |
| Post PK | 4 | 14 | 5 | 4 |
| Chemical injury | 1 | 2 | 5 | 2 |
| Post VR surgery | 2 | 0 | 1 | 2 |
| Post keratitis | 1 | 1 | 2 | 2 |
| Neurotrophic ulcer | 3 | 1 | 5 | 3 |
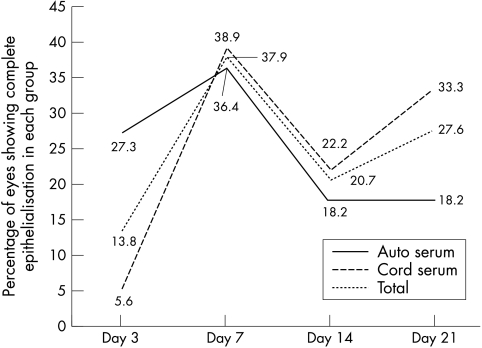
The trend lines for the percentage of eyes that showed complete healing of the epithelial defect in the two groups at each of the follow up days are shown in Figure 1. These were not significantly different. The median time for closure of epithelial defects was 12.32 days in the autologous serum group (Fig 2A–C) and 16.64 days in the cord serum group (Fig 3A–C) (p = 0.18). However, the percentage decrease in size of the epithelial defect as measured in terms of area and perimeter was different in the two groups, being significantly more in the cord serum group (Table 4).
Figure 1.
Trend lines for percentage of eyes in each group showing complete re-epithelialisation at each follow up day.
Figure 2.
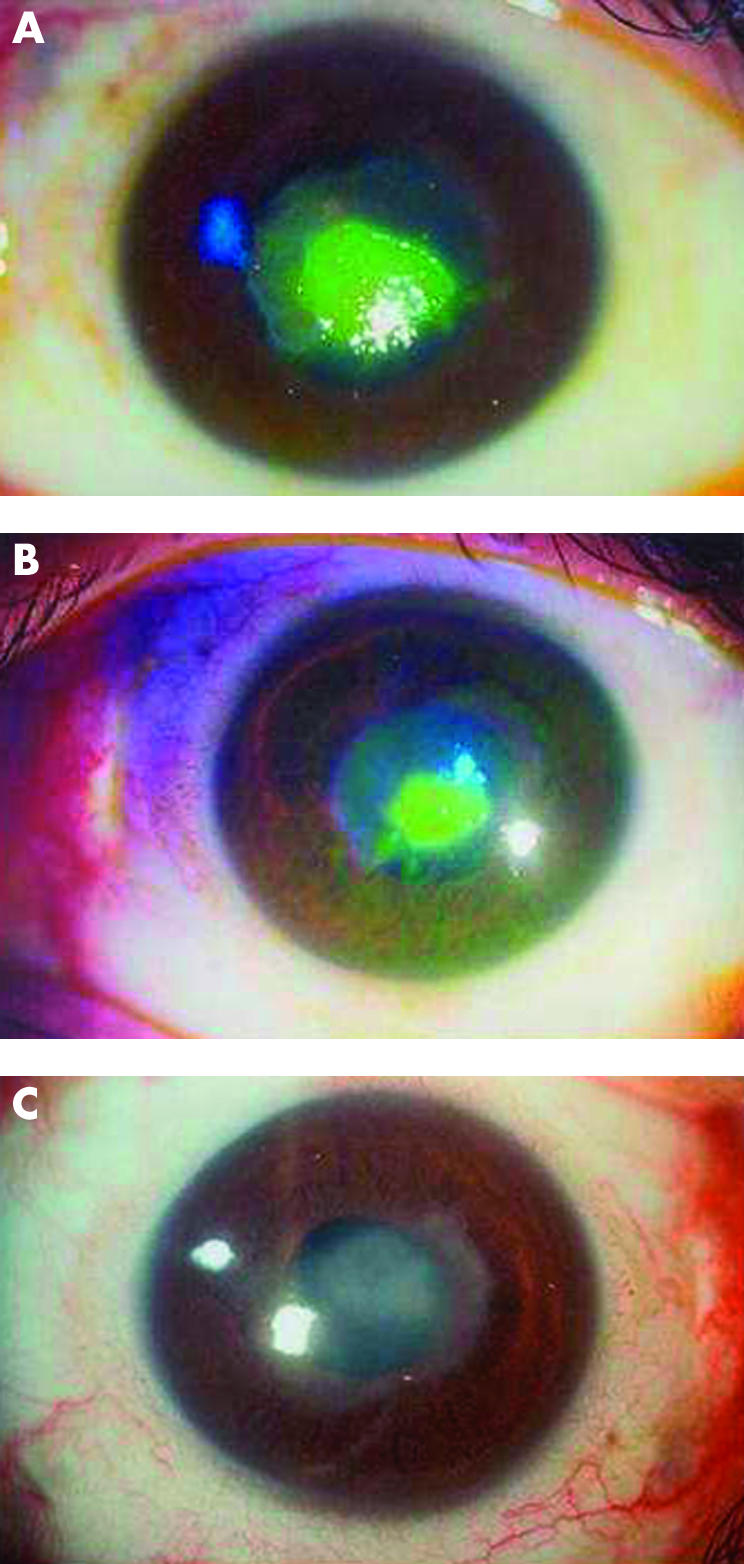
Healing of a persistent epithelial defect on autologous serum on day 0 (A), day 7 (B) and day 14 (C).
Figure 3.
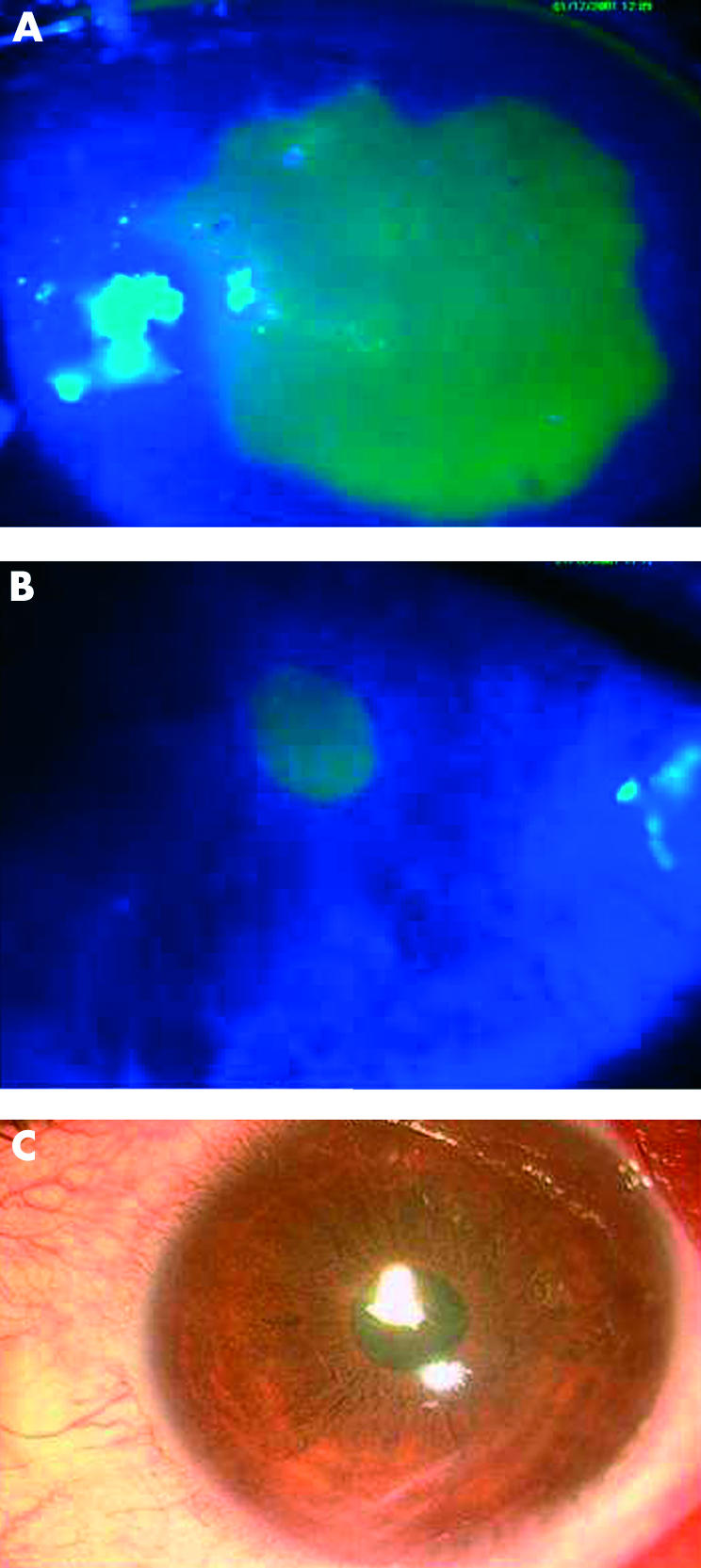
Healing of a persistent epithelial defect on umbilical cord serum on day 0 (A), day 7 (B) and day 14 (C).
Table 4.
Rate of healing in the two groups using different parameters
| Outcome variables | Percentage decrease | Statistical significance (Wilcoxon rank sum test) | ||
| Autologous serum group (n = 29) | Umbilical cord serum group (n = 31) | Z value | p Value | |
| Median (range) | Median (range) | |||
| Horizontal diameter | ||||
| Day 3 | 8.3 (−133.3–56.5) | 3.0 (−220.0–57.1) | 0.50 | 0.614 |
| Day 7 | 10.0 (−100.0–100.0) | 24.1 (−14.3–100.0) | 1.16 | 0.244 |
| Day 14 | 11.7 (–60.0–37.5) | 30.4 (–6.1–100.0) | 1.81 | 0.068 |
| Day 21 | 13.1 (–55.0–50.0) | 33.9 (–7.9–100.0) | 1.37 | 0.184 |
| Vertical diameter | ||||
| Day 3 | 9.5 (–210.0–86.8) | 3.2 (–960.0–33.33) | 0.49 | 0.618 |
| Day 7 | 9.8 (–77.1–100.0) | 22.5 (–100.0–100.0) | 0.90 | 0.364 |
| Day 14 | 9.3 (–82.7–53.3) | 23.5 (–100.0–100.0) | 0.96 | 0.333 |
| Day 21 | 11.9(–85.7–53.3) | 20.0 (–100.0–100.0) | 0.35 | 0.724 |
| Area | ||||
| Day 3 | 0.7 (–657.6–82.3) | 11.5 (–1351.6–65.1) | 0.49 | 0.619 |
| Day 7 | 2.9 (–66.5–14.9 ) | 53.0 (–48.0–100.0) | 2.36 | 0.018 |
| Day 14 | 13.8(–42.9–32.5) | 63.0 (0.0–86.7) | 2.72 | 0.013 |
| Day 21 | 6.7 (–41.7–33.1) | 62.7 (0.0–79.6) | 2.57 | 0.009 |
| Perimeter | ||||
| Day 3 | 1.2 (–589.7–73.1) | 13.2 (–366.85–66.34) | 0.59 | 0.558 |
| Day 7 | 3.1 (–38.2–14.3) | 32.5 (–48.6–100.0) | 2.69 | 0.007 |
| Day 14 | 6.5 (–18.4–30.5) | 48.5 (0.0–61.3) | 2.30 | 0.021 |
| Day 21 | 2.7 (–17.8–30.3) | 48.9 (0.0–75.5) | 2.24 | 0.024 |
| Major axis (automated) | ||||
| Day 3 | 3.3 (–121.5–44.6) | 11.3 (–209.5–55.1) | 0.00 | 1.000 |
| Day 7 | 4.8 (–93.8–16.4) | 65.0 (–17.4–100.0) | 2.86 | 0.004 |
| Day 14 | 9.5 (–48.3–26.6) | 62.7 ( 0.0–85.2) | 2.32 | 0.020 |
| Day 21 | 5.6 (–47.9–25.5) | 67.1 (0.0–75.1) | 2.41 | 0.015 |
| Minor axis (automated) | ||||
| Day 3 | 2.9 (–214.4–74.2) | 17.2 (–642.8–41.7) | 0.29 | 0.770 |
| Day 7 | 2.6 (–10.9–23.7) | 54.2 (–75.2–100.0) | 2.64 | 0.008 |
| Day 14 | 4.9 (–4.9–35.8) | 51.0 (–89.4–70.7) | 1.51 | 0.129 |
| Day 21 | 6.2 (–4.9–28.4) | 41.7 (–75.2–64.8) | 1.34 | 0.180 |
On further follow up of 1 month of the 29 patients who had healed, 21 patients remained on follow up and none of them showed a recurrence of the epithelial defect. Among the 31 patients who did not show complete healing at day 21 on either therapy 20 underwent a temporary lateral tarsorrhaphy, with a subsequent healing of the epithelial defect. Nine patients underwent an amniotic membrane transplantation (AMT) and at last follow up eight of those had a complete re-epithelialisation of the corneal surface. One patient who underwent AMT was lost to follow up. The remaining two patients in the failure group developed a perforation, one post-traumatic and the other following bacterial keratitis (developed 1 month after the patient stopped using serum). Both these patients underwent therapeutic penetrating keratoplasty. None of our patients from either group reported any adverse event during the therapy.
DISCUSSION
Healing of a corneal epithelial defect occurs in phases and is completed when the regenerated epithelium forms adhesions to the underlying stroma.1,11 Common treatments available include tear supplementation5 and bandage contact lenses,12 tarsorrhaphy,13 and elimination of potentially epitheliotoxic preservatives and drugs.5,14 The newer treatments of treatment are amniotic membrane transplantation,15 allolimbal/autologous limbal cell transplant,4,16,17 topical fibronectin,18,19 growth factors,20–24 tetracycline,25 acetylcholine and propolis extract,26 and a new biomaterial CxGelsix.27 Treatment by reconstruction with cultured corneal epithelial cells28 has also been tried.
Amniotic membrane transplantation has been shown to be effective in persistent epithelial defects resistant to conventional medical therapy.15,17 A possible mechanism of action is that it is a source of growth factors essential to the proliferation of the residual stem cell population.29 Likewise, autologous serum has been used in such circumstances to supply the ocular tissue with epidermal growth factor, vitamin A, transforming growth factor β, fibronectin, and other cytokines,7,30 and differentially modulates the proliferation of corneal and limbal cells.7,31
The recently recognised role of growth factors and the success of the amniotic membrane transplantation in the healing of persistent epithelial defect was the basis of our study. The cornea produces multiple growth factors such as epidermal growth factor (EGF) and fibroblast growth factor (FGF), neurotrophic growth factor which contribute to the maintenance of a healthy epithelial surface and its regeneration.23–24,32
Tsubota et al7 studied the in vitro cell migration in different concentrations of fetal calf serum and found them to be significantly higher than in serum free control media. The fact that fetal calf serum enhances cell migration and is very likely to be different from adult serum in terms of growth factors6,7 warranted an evaluation.
The reason why serum is of benefit to the ocular surface is not entirely clear but probably owes its efficacy to the presence of factors such as EGF, vitamin A, acidic and basic FGF, fibronectin, nerve growth factor, substance P, antiproteases like α2 macroglobulin, and enhanced mucin expression due to the serum.7,30 Umbilical cord serum presumably has these factors in a higher concentration although a formal comparison of the exact levels of these factors in both the sera does not find a mention in the literature. The support for this presumption comes from the fact that umbilical cord serum has been used extensively as a source of stem cells in treating haematopoietic malignancies and fetal calf serum is used commonly for cell and tissue cultures.6–8 A complete analysis of the growth factors in both cord blood serum and autologous serum would have eliminated all speculation but could not be accomplished for lack of facilities.
Our findings corroborated our hypothesis as we found a significantly higher rate of healing in the cord serum group when measuring the areas and perimeters on serial photographs in an automated manner using the software Image Pro Plus, thereby eliminating all measure of human error and accounting for irregular healing patterns.11
The mechanism of action of umbilical cord serum is likely to be the same as that of autologous serum, the difference being probably a higher concentration of the growth factors, which may in fact stimulate a faster growth of stem cells and hence lead to a faster re-epithelialisation. Another important observation was that the epithelial defects increased in size over the first 3 days as evident from Table 4 (the ranges becoming negative). This biphasic response was similar to that reported by Dua et al.11 Despite a faster rate of healing in the umbilical cord serum group the median time to closure of the epithelial defects was less in the autologous serum group probably because the epithelial defects treated with cord serum showed a considerably larger increase in size initially when compared to those in the autologous serum group.
In a report of 16 cases with persistent epithelial defect Tsubota et al7 reported a 43.8% healing within 2 weeks and a further 18.8% within a month on autologous serum drops. A similar case series by Poon et a.30 shows a healing in nine out of 15 cases with autologous serum drops in a mean period of 29 days with a recurrence in five eyes after cessation of serum therapy. To the best of our knowledge this is the first randomised controlled clinical trial evaluating the efficacy of umbilical cord serum. Our healing rate is comparable and a little faster, probably because of the washout given to the epithelial defects before commencement of the medication and non-administration of any epitheliotoxic drugs concurrently. Our aetiological groups and their distribution in the two treatment groups were heterogeneous; however, their distribution was not statistically different in the two groups of patients. As the basic cause of epithelial defect is different in various disease groups, it would be ideal to study each disease group separately.
Our study corroborates the fact that serum, either umbilical cord or autologous as a 20% solution, is a safe and non-toxic preparation that can be easily prepared and used.
The risk of allergies and possibilities of transmitting parenterally transmitted organisms must also be kept in mind when using umbilical cord serum apart from the legal and ethical issues. A routine testing of the mothers and a rapid test on the sera for viral contaminants is warranted. When the event to the time between the testing of the mother for HIV and the preparation of cord serum from the placental blood is more than 6 months, HIV testing should be undertaken again at the time of serum delivery to account for the window period of the infection.
The recurrence of epithelial defects in patients with diagnoses similar to ours is known.1,7,30 However it is noteworthy that none of the 21 patients who were on follow up for another month after complete re-epithelialisation showed a recurrence even at 3 months of follow up. Although the risk of a recurrence of epithelial defects in the patients lost to follow up cannot be denied, the trend in our group of patients does not entirely corroborate the theoretical risk. Also, it would have been worthwhile to evaluate the corneal sensitivity before and after therapy to find if there is a substantial difference or recovery with re-epithelialisation.
To conclude, we recommend that umbilical cord serum is an effective means of promoting epithelialisation and can be safely used.
Acknowledgments
We thank Samir A Melki, MD, PhD, director, Boston Cornea Centre for the idea of using cord blood serum for persistent epithelial defects.
REFERENCES
- 1.Dua HS, Gomes JAP, Singh A. Corneal epithelial wound healing. Br J Ophthalmol 1994;78:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S- H, Tseng SCG. Amniotic membrane transplantation for peristent epithelial defects with ulceration. Am J Ophthalmol 1997;123:303–12. [DOI] [PubMed] [Google Scholar]
- 3.Berman M. The pathogenesis of corneal epithelial defects. Acta Ophthalmol Scand 1989;67:55–64. [DOI] [PubMed] [Google Scholar]
- 4.Dua HS, Blanco AA. Allo-limbal transplantation in patients with limbal stem cell deficiency. Br J Ophthalmol 1999;83:414–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfister RR. Clinical measures to promote corneal epithelial healing. Acta Opthalmol Scand 1992;70:73–83. [DOI] [PubMed] [Google Scholar]
- 6.Borderie VM, Mourra N, Laroche L. Influence of fetal calf serum, fibroblast growth factors, and hepatocyte growth factor on three-dimensional cultures of human keratocytes in collagen gel matrix. Graefes Arch Clin Exp Ophthalmol 1999;237:861–9. [DOI] [PubMed] [Google Scholar]
- 7.Tsubota K, Goto E, Shimmura S, et al. Treatment of persistent epithelial defect by autologous serum application. Ophthalmology 1999;106:1984–9. [DOI] [PubMed] [Google Scholar]
- 8.Katz LEL, Cohen P. Growth factor regulation of fetal growth. In: Polin RA, Fox WW, eds. Fetal and neonatal physiology Philadelphia: WB Saunders, 1998:2401–4.
- 9.Han VKM. Fetal growth and its regulation. In: Rodeck CH, Whittle MJ, eds. Fetal medicine, basic sciences and clinical practice London: Churchill Livingstone, 1999:127–39.
- 10.Mukerji N, Sinha R, Vajpayee RB. Role of autologous serum in persistent epithelial defects. Br J Ophthalmol 2002;86:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dua HS, Forrester JV. Clinical patterns of corneal epithelial wound healing. Am J Ophthalmol 1987;104:481–9. [DOI] [PubMed] [Google Scholar]
- 12.Ali Z, Insler MS. A comparison of therapeutic bandage lenses, tarsorrhaphy, antibiotic and hypertonic saline on corneal epithelial wound healing. Ann Ophthalmol 1986;18:22–4. [PubMed] [Google Scholar]
- 13.Panda A, Pushker N, Bageshwar LM. Lateral tarsorrhaphy: is it preferable to patching? Cornea 1999;18:299–301. [DOI] [PubMed] [Google Scholar]
- 14.Pfister RR, Burnstein N. The effects of ophthalmic drugs, vehicles, and preservatives on corneal epithelium: a scanning electron microscope study. Invest Opthalmol Vis Sci 1976;15:246–59. [PubMed] [Google Scholar]
- 15.Blanco AA, Pillai CT, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol 1999;83:399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan DTH, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology 1996;103:29–36. [DOI] [PubMed] [Google Scholar]
- 17.Tseng SCG, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol 1998;116:431–41. [DOI] [PubMed] [Google Scholar]
- 18.Gordon JF, Johnson P, Musch DC and the Chiron Vision Fibronectin Study Group. Topical fibronectin ophthalmic solution in the treatment of persistent defects of the corneal epithelium. Am J Ophthalmol 1995;119:281–7. [DOI] [PubMed] [Google Scholar]
- 19.Phan TM, Foster CS, Boruchoff SA, et al. Topical fibronectin in the treatment of persistent corneal epithelial defects and trophic ulcers. Am J Ophthalmol 1987;104:494–501. [DOI] [PubMed] [Google Scholar]
- 20.Bonini S, Lambiase A, Rama P, et al. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology 2000;107:1347–51. [DOI] [PubMed] [Google Scholar]
- 21.Lambiase A, Manni L, Bonini S, et al. Nerve growth factor promotes corneal healing: structural, biochemical and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci 2000;41:1063–9. [PubMed] [Google Scholar]
- 22.Sotozono C, Inatomi T, Nakamura M, et al. Keratinocyte growth factor accelerates corneal epithelial wound healing in vivo. Invest Ophthalmol Vis Sci 1995;36:1524–9. [PubMed] [Google Scholar]
- 23.Imanishi J, Kamiyama K, Iguchi I, et al. Growth factors: importance in wound healing and maintenance of transparency of the corneas. Prog Retinal Eye Res 2000;19:113–29. [DOI] [PubMed] [Google Scholar]
- 24.Chikama T, Fukuda K, Morigishe N, et al. Treatment of neurotrophic keratopathy with substance-P derived peptide (FGLM) and insulin-like growth factor I. Lancet 1998;351:1783–4. [DOI] [PubMed] [Google Scholar]
- 25.Ralph RA. Tetracyclines and the treatment of corneal stromal ulceration. Cornea 2000;19:274–277. [DOI] [PubMed] [Google Scholar]
- 26.Ozturk F, Kurt E, Inan UU, et al. The effects of acetylcholine and propolis extract on corneal epithelial wound healing in rats. Cornea 1999;18:466–71. [DOI] [PubMed] [Google Scholar]
- 27.Leksul M, Burrows R, Kublin CL, et al. Cx GELSIX: A novel preparation of type VI collagen with possible use as biomaterial. Cornea 2000;19:194–203. [DOI] [PubMed] [Google Scholar]
- 28.Pellegerini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997;349:990–3. [DOI] [PubMed] [Google Scholar]
- 29.Dua HS, Blanco AA. Amniotic membrane transplantation. Br J Ophthalmol 1999;83:748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poon AC, Geerling G, Dart JKG, et al. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol 2001;85:1188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruse FE, Tseng SCG. Serum differentially modulates the clonal growth and differentiation of cultured limbal and corneal epithelium. Invest Ophthalmol Vis Sci 1998;34:2976–89. [PubMed] [Google Scholar]
- 32.Tripathi BJ, Kwait PS, Tripathi RC. Corneal growth factors: a new generation of ophthalmic pharmaceuticals. Cornea 1990;9:2–9. [PubMed] [Google Scholar]