Abstract
Aim: To determine the effects of single doses of β radiation on the wound healing functions of human Tenon’s capsule fibroblasts (hTf).
Methods: hTf were grown in tissue culture and irradiated with β radiation using a strontium 90 source. The effects of β radiation on fibroblast migration was studied using microporous transwell membranes. The effects of radiation on fibroblast contraction was investigated using a fibroblast populated collagen gels model. Production of extracellular matrix molecules (collagen I, collagen III, and fibronectin) by monolayers of irradiated fibroblasts was quantified for 14 days following single doses of β radiation.
Results: Growth inhibiting doses of β radiation did not inhibit fibroblast migration or contraction at any time point. Levels of soluble fibronectin from irradiated populations were significantly reduced after >500 cGy β radiation. Collagen I and III levels were not reduced after any dose of radiation, and increased following treatment with 1000 cGy β radiation.
Conclusions: Growth arresting doses of β radiation have unique effects on the wound healing behaviour of human Tenon’s capsule fibroblasts. There was no significant effect on cellular migration or contraction, but ECM production was altered. Fibronectin production was inhibited following higher radiation doses, and collagen I and III production increased after 1000 cGy. The effects of single doses of β radiation on ocular fibroblast wound healing behaviour are very different from those of 5-fluorouracil and mitomycin C, and these differences may be exploited clinically in the regulation of wound healing after glaucoma filtration surgery.
Keywords: radiation, fibroblasts, wound healing
The nature of the postoperative healing response that occurs in the subconjunctival tissues following filtration surgery is increasingly recognised to be fundamental, both to the survival of the filtration bleb as well as in determining the degree of pressure reduction that is achieved after surgery. This healing response consists of a cascade of cellular and biochemical events leading to the activation, migration, and proliferation of local fibroblasts, together with the deposition of new extracellular matrix and scar formation. Over recent years, treatments that modulate the subconjunctival healing response, particularly 5-fluorouracil (5-FU) and mitomycin C (MMC), have been shown to be extremely effective clinically at preventing bleb failure, but the use of these liquid agents has been associated with complications from inadvertent extraocular and intraocular leakage, as well as with sight threatening complications including hypotony and endophthalmitis.1,2 These complications appear to be particularly associated with the formation of thin avascular cystic blebs after antimetabolite treatment, and this represents a significant problem in the long term management of glaucoma patients.
Single doses of β radiation delivered from a solid state strontium 90 source have previously been shown to exert a powerful antiproliferative effect on ocular fibroblasts in vitro,3 as well as being clinically effective at enhancing bleb survival after trabeculectomy in patients with congenital glaucoma.4 The possibility that focally delivered β radiation treatment may offer an effective alternative to the use of liquid antimetabolites has prompted our group to further investigate the effects that such treatments have on the overall wound healing functions of human Tenon’s capsule fibroblasts (hTf). We have previously reported that single doses of β radiation up to 1000 cGy effectively induce a rapid onset and sustained period of growth arrest in hTf,5 and have shown this to be associated with an increase in cellular p53 levels, a nuclear phosphoprotein which is closely involved in the regulation of cell cycling and cell death in many cells.6
Although cell proliferation is one of the central cellular responses in wound healing, hTf also perform other important functions in healing wounds including migration, contraction, and extracellular matrix (ECM) production.7 Previous work has shown that treatment with 5-FU and MMC significantly inhibits many of these cellular processes,8,9 indicating that the clinical effect is the result of a global inhibition in hTf wound healing functions. In this paper we have investigated the effects of growth arresting doses of β radiation on these wound healing processes to determine if radiation treatment differs in its effects, as we hope that an increased understanding of the cellular effects of this novel anti-scarring treatment may enhance development of its clinical use.
MATERIALS AND METHODS
Cell culture
Human Tenon’s fibroblasts were propagated from donor explants using a previously described protocol.10 Cultures were passaged at subconfluence, and the cells used between passages 3 and 7 for experimentation. The tenets of the Declaration of Helsinki were followed, and institutional human experimentation ethics committee approval was granted. Each experiment was repeated a minimum of three times to ensure reproducibility.
Cell migration
Fibroblast migration was studied with Costar transwells (Corning Costar Corporation, Cambridge MA, USA) using a previously described protocol.9 Briefly, subconfluent hTf were placed in the inner chamber of the wells in serum free medium and allowed to settle. Control cells remained unirradiated, while test populations were treated with single doses of β radiation ranging between 500 and 2000 cGy (positive control transwells were sham treated for a period equal to the longest time irradiated transwells were removed from the plate, negative controls were migrated against serum free DMEM). Migration was assayed using DMEM containing 20% fetal calf serum in the outer chamber as this has previously been shown to give a maximal chemotactic response using a chequerboard analysis. At each time point, test and control transwell membranes were fixed with methanol, air dried, and stained with Mayer’s haematoxylin (BDH). Non-migrated cells were removed from the upper surface of the membranes, and the mean number of hTf migrating per high power field (×400) was determined using triplicate membranes for each of the treatment groups and controls. The effects of β radiation on hTf migration were studied at 0, 3, 7, and 14 days following irradiation. At each time point, the mean number of migrated cells per field for each treatment group was compared to the time matched positive and negative controls, and the results analysed using a one way analysis of variance (ANOVA), correcting the significance levels using the Bonferroni test. A probability (p) value of less than 0.05 was considered as indicating significance.
Cell contraction
A modification of a previously described free floating fibroblast populated collagen lattice (FPCL) model of in vitro contraction was used to study fibroblast mediated wound contraction.9 Subconfluent hTf were irradiated with doses of 500,1000, or 2000 cGy β radiation, with control populations remaining unirradiated. Lattices were prepared by adding irradiated or control hTf to a mixture of rat tail collagen I (C8897, Sigma) dissolved in 0.1% glacial acetic acid and concentrated medium stock solution as previously described. Each gel contained 26 000 hTf as this had been found to give the optimum dynamic range for the assay in previous standardisation experiments. After setting, the FPCL were fed with fresh DMEM/10%FCS, and the gels detached from the base of the well plate. Triplicate FPCL were cast for each test and control group. The area of test and control FPCL was measured on days 0, 3, 7, 10, and 14 by serial photographs and analysed using a Kurta 1212 Summasketch board (Kurta, Phoenix, AZ, USA), and Sigmascan software (Jandel Scientific, CA, USA) using the technique previously described.9 The mean area of the gels at each time point was determined for each of the treatment and control groups, and compared using a one way ANOVA with Bonferroni modification as before.
Extracellular matrix (ECM) assay
Conditioned medium samples
The effects of β radiation on fibroblast ECM production were assessed by quantifying the levels of the ECM molecules collagen I, collagen III, and fibronectin present in 24 hour conditioned medium samples collected from control and irradiated fibroblast populations at various time points following irradiaiton. hTf were used in confluent culture, and test populations were irradiated in situ on day 0 with doses of 250, 500, or 1000 cGy β radiation as before (control populations remained unirradiated). The growth medium was changed every 3 days to ensure all cells remained viable in confluent culture. At each time point studied, triplicate test and control wells were washed with PBS and the medium replaced with 110 μl of serum free DMEM containing 3% bovine serum albumin (for the collagen assays, the medium also contained 0.15 mM ascorbic acid). After 24 hours 100 μl samples of this conditioned medium were collected from each monolayer and stored at −70°C till needed for analysis. After collection of each conditioned medium sample (fibronectin, collagen I, and collagen III assays), the cells in each monolayer were stained with Mayer’s stain and counted with a high power microscope to allow the results to be standardised for cell number.
Fibronectin assay
Soluble fibronectin levels in conditioned medium samples were measured using an ELISA test previously described by Khaw et al.8 Briefly, conditioned medium samples (diluted 1:100 in PBS to give levels within the linear range of the assay) were added to the wells of an ELISA plate that had been precoated with anti-human fibronectin (Dako A245). Bound fibronectin was then detected using Dakopatts peroxidase conjugated rabbit anti-human fibronectin antibody (Dako P246) and developed with O-phenylenediamine dihydrochloride (OPD, Sigma) as above. A standard curve of purified human fibronectin (Sigma) was also generated with each assay, and the colourimetric change analysed as before.
Collagen I and III assay
Collagen assays were performed using a direct ELISA test which we have developed and recently described.8 Briefly, test wells in 96 well ELISA plates were coated with conditioned medium samples, and then the bound collagen I and III detected using goat anti-human collagen type I (Southern Biotechnology) or goat anti-human collagen type III (Southern Biotechnology), respectively, as primary antibodies. The bound primary antibody was labelled with peroxidase conjugated rabbit anti-goat IgG (Eurogenetics) and this was developed colorimetrically using OPD and a Titertek Plus MS2 microplate reader (ICN Flow, Bucks, UK). Standard curves of purified human collagen I and III were simultaneously generated and an equation for the standard curve determined with a polynomial trendline using computer software (Microsoft Excel). The level of collagen I and III in conditioned medium samples was determined using this standard curve equation, and expressed as the mean (SE) of triplicate wells. Results were corrected for cell number and analysed using a one way analysis of variance (ANOVA) as before.
RESULTS
Migration
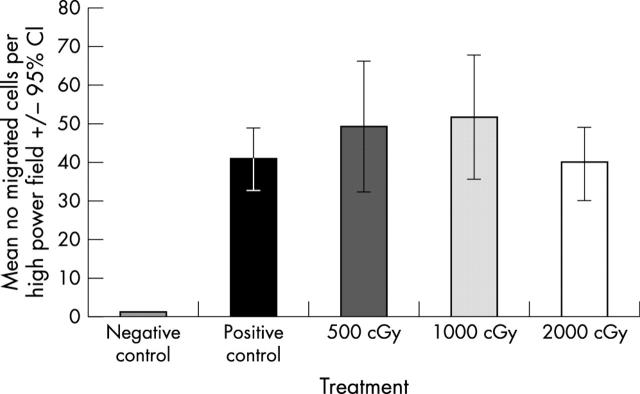
At day 0 (16 hours after treatment) there was no significant difference in the number of migrated fibroblasts in any of the irradiated populations compared with (unirradiated) positive control fibroblasts (p = 0.2445, fig 1). Furthermore, no significant difference was demonstrated between the number of migrated cells in the treatment or positive control groups at any of the later time points studied (p = 0.5 at day 3, p = 0.82 and p = 0.132 at days 3, 7, and 14 respectively, data not shown).
Figure 1.
Effect of radiation on fibroblast migration at 16 hours (day 0).
Contraction
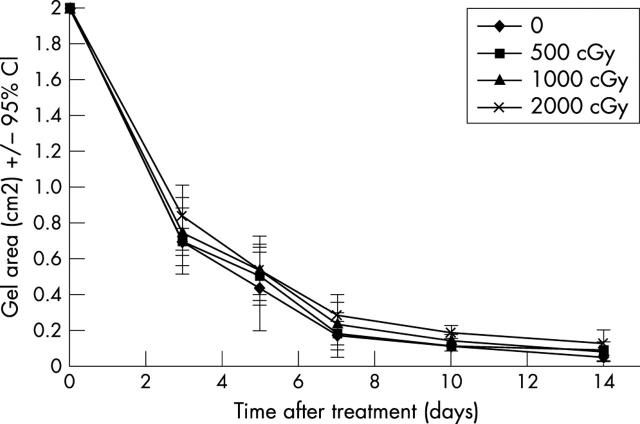
Unirradiated control lattices showed rapid contraction over the first 5 days, contracting by an average of 74% over this period, and thereafter continued to reduce in size up to day 10, although at a slower rate. There was no significant difference in the rate or degree of gel contraction in any of the irradiated populations compared to the time matched unirradiated controls (fig 2) (p = 0.4334 day 3, p = 0.9117 day 5, p = 0.8367 day 7, p = 0.4302 day 9, p = 0.259 day 12 and p = 0.775 day 14). As these experiments involved irradiating the hTf while in situ within the gels, and in order to demonstrate that the collagen matrix itself was not attenuating the radiosensitivity of the cells, the experiments were also repeated with fibroblasts that had been irradiated before casting into the gels, but again no significant difference was found between irradiated groups and the controls (data not shown).
Figure 2.
Effect of radiation on matrix contraction.
ECM production
Fibronectin
The level of soluble fibronectin in conditioned medium samples from control cells increased significantly over the time course of the experiment (p<0.05). For the irradiated hTf, fibronectin levels from hTf treated with 1000 cGy showed a significant reduction compared to control cells at day 1 (p<0.05), but there was no significant difference comparing lower radiation doses and controls. At day 7 fibronectin levels from fibroblasts irradiated with 500 or 1000 cGy were significantly lower than the levels measured from control cells (p<0.05), and this trend appeared to continue up to day 14, although it was not confirmed statistically (fig 3A).
Figure 3.
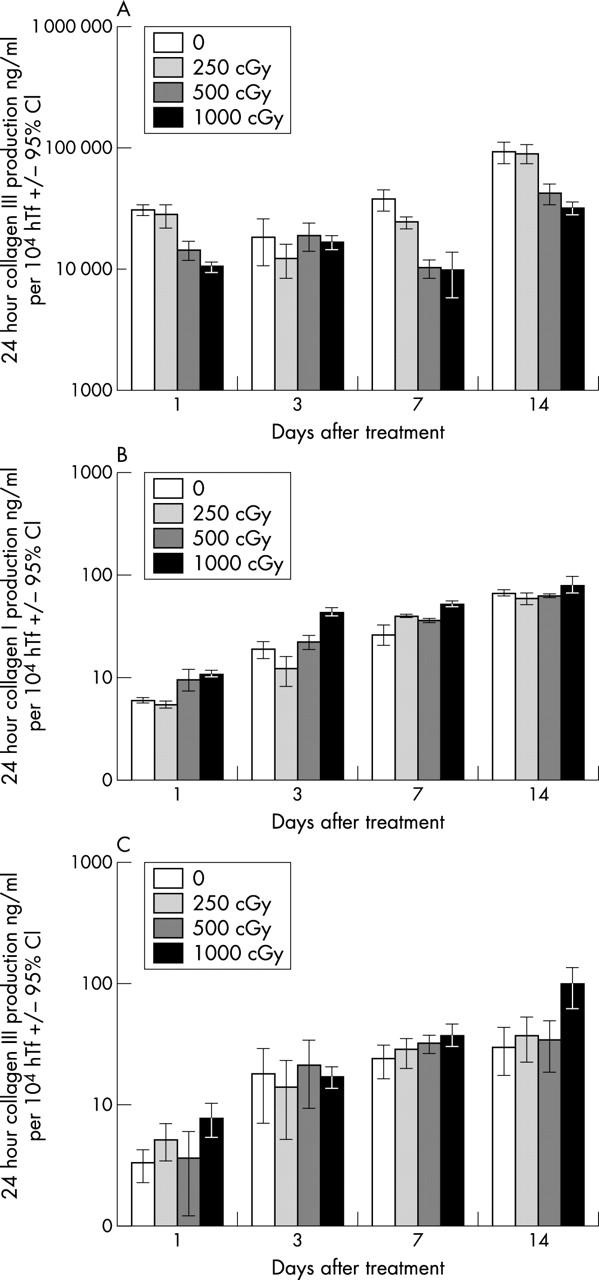
Effect of radiation on ECM production. (A) Fibronectin; (B) collagen I; (C) collagen III.
Collagen I
The level of soluble collagen I in conditioned medium samples from all treatment and control populations showed a progressive increase over time, with a significant increase above day 1 levels detected in all groups by day 14 (p<0.05). The soluble collagen I levels from maximally irradiated populations (1000 cGy) were significantly greater than those from the unirradiated control cells at days 1, 3, and 7 (p<0.05). At day 14, although this trend appeared to continue, this was not confirmed statistically. The levels of collagen I from cell populations treated with lower doses of β radiation, although also appearing to increase, were not significantly different from the time matched controls (fig 3B).
Collagen III
As with collagen I, the level of soluble collagen III in conditioned medium samples increased over time for all the treatment groups (p<0.05). The collagen III levels from the maximally irradiated group (1000 cGy) were significantly greater than from the unirradiated controls at days 1, 7, and 14. The levels of collagen III also appeared to be increased in the lower radiation groups, but this was not confirmed statistically (fig 3C).
DISCUSSION
The use of focal applications of β radiation to prevent scarring following filtration surgery is not a new concept, with the earliest case reports dating from the 1940s.11,12 In 1991, Miller and Rice reported that peroperative treatment with 750 cGy β radiation significantly enhanced bleb survival and improved IOP control following trabeculectomy in children.4 A more recent paper by Rehman et al also reported the effect of preoperative β radiation on the outcome of filtration surgery, with successful IOP control in 90% of the irradiated eyes; although this was not significantly greater than in their control group.13 Our clinical experience of this treatment to date has demonstrated that focal β radiation is a safe, well tolerated, and effective treatment for preventing bleb failure, avoiding many of the complications associated with the liquid antimetabolites. Recently, interest in the use of alternative strategies for manipulating bleb healing has grown, particularly for use in the Third World where the use of liquids which need refrigeration may prove logistically difficult. Our group has been investigating the cellular effects of such single doses of β radiation in an attempt to better understand the differences in effect between this and other antimetabolite agents, as we hope this will direct and enhance development of this novel treatment for altering healing after glaucoma filtration surgery.
Previous in vitro studies of both animal and human ocular fibroblasts have demonstrated that single doses of β radiation can effectively inhibit fibroblast proliferation by inducing a rapid onset and sustained period of growth arrest.5 Similarly, short exposures to 5-FU and MMC induce a similar growth arrest response in fibroblasts, although interestingly MMC has also been shown to induce apoptosis in fibroblasts,14 and this greater response may be very relevant in the genesis of thin, avascular blebs. For this paper the doses of radiation used in these experiments were clinically relevant growth arresting doses of β radiation, which are not associated with increased levels of cell death.5
Fibroblast migration is a central process in a healing wound, increasing both the number of fibroblasts in the wound, as well as generating contractile forces that assist in wound closure.15 We have shown that for up to 14 days following treatment, irradiated fibroblasts retain the same migratory capacity as time matched controls, demonstrating that β radiation at growth arresting doses does not interfere with the ability of hTf to migrate. This finding is supported by data from Orredson et al who noted that there was no change in either the directed or random movement of rabbit fibroblasts following treatment with 2000 cGy x rays,16 and this effect is strikingly different from the effects of 5-FU and MMC, which both inhibit hTf migration.8
Occleston et al reported that growth arresting doses of 5-FU and MMC significantly inhibit fibroblast mediated wound contraction.9 Interestingly, although it is known that irradiated skin wounds show a delay in wound contraction, our results have demonstrated that irradiated fibroblasts are unaltered in their contractile ability compared with time matched controls. This suggests that the tissue effect reflects a reduction in total wound cellularity, rather than a specific inhibition of contractile function. This finding is further supported by Yanase et al, who reported that irradiation of oral fibroblasts with x rays (200 to 1000 cGy) immediately after casting in collagen gels had no effect on either the rate or degree of contraction of the gels.17
The fibroblasts in healing wounds are also important in synthesising new ECM molecules including fibronectin, which acts as a primary scaffold for cell attachment and migration, and collagen I and III which increase wound strength.18,19 Our group has previously shown that hTf treated with growth arresting doses of 5-FU or MMC show a long term reduction in the production of fibronectin, collagen I and collagen III.8 In contrast with this effect, these experiments have shown that while treatment with growth arresting doses of β radiation causes a significant reduction in fibronectin production, interestingly, the levels of collagen I and III from irradiated cell populations were found to be increased, suggesting that higher doses of radiation may actually increase cellular collagen production. This novel finding is supported by recent evidence that preterminal cellular differentiation may be induced in fibroblasts following radiation treatment,20 and suggests that non-lethal radiation exposure may induce the fibroblasts to transform into a matrix secreting cell, and this may be an important factor in the development of post-radiation fibrosis.
In conclusion, we have demonstrated that unlike the effects of 5-FU and MMC, growth arresting doses of β radiation do not alter the ability of human Tenon’s fibroblasts to migrate or contract. Furthermore, although fibronectin production is inhibited by β radiation treatment, we have demonstrated that collagen I and III production is not reduced, and may actually be increased after higher doses of radiation. It appears that β radiation acts as an effective antiproliferative treatment rather than as a global inhibitor of fibroblast wound healing behaviour, and understanding and exploiting this difference may be extremely important in developing its clinical use.
Acknowledgments
This work was supported in part by the TFC Frost Charitable Trust, the International Glaucoma Association, Guide Dogs for the Blind, and the Wellcome Trust.
REFERENCES
- 1.Jampel HD, Pasquale LR, Dibernardo C. Hypotony maculopathy following trabeculectomy with mitomycin C. Arch Ophthalmol 1992;110:1049–50. [DOI] [PubMed] [Google Scholar]
- 2.Higginbotham EJ, Stevens RK, Musch DC, et al. Bleb-related endophthalmitis after trabeculectomy with mitomycin. Ophthalmology 1996;103:650–6. [DOI] [PubMed] [Google Scholar]
- 3.Khaw PT, Ward S, Grierson I, et al. Effect of beta radiation on proliferating human Tenon’s capsule fibroblasts. Br J Ophthalmol 1991;75:580–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MH, Rice NSC. Trabeculectomy combined with beta irradiation for congenital glaucoma. Br J Ophthalmol 1991;75:584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constable PH, Crowston JG, Occleston NL, et al. Long term growth arrest of human Tenon’s fibroblasts following single applications of β radiation. Br J Ophthalmol 1998;82:448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kastan MB, Onyekwere O, Sidransky D, et al. Participation of p53 protein in the cellular response to DNA damage. Cancer Res 1991;51:6304–11. [PubMed] [Google Scholar]
- 7.Clark RAF, Henson PM. The molecular and cellular biology of wound repair, 1 ed. New York: Plenum Press, 1988.
- 8.Occleston NL, Daniels JT, Tarnuzzer RW, et al. Single exposures to antiproliferatives: long term effects on ocular fibroblast wound healing behaviour. Invest Ophthal Vis Sci 1997;38:1998–2002. [PubMed] [Google Scholar]
- 9.Occleston NL, Alexander RA, Mazure A, et al. Effects of single exposures to antiproliferative agents on ocular fibroblast mediated collagen contraction. Invest Ophthalmol Vis Sci 1994;35:3681–90. [PubMed] [Google Scholar]
- 10.Joseph JP, Grierson I, Hitchings RA. Normal rabbit aqueous humour, fibronectin and fibroblast conditioned medium are chemoattractant to Tenon’s capsule fibroblasts. Eye 1987;1:585–92. [DOI] [PubMed] [Google Scholar]
- 11.Iliff CE. Surgical control of glaucoma in the negro. Am J Ophthalmol 1944;27:731–8. [Google Scholar]
- 12.Burnam CF, Neill W. Use of beta ray of radium applicator. South Med J 1940;33:279–84. [Google Scholar]
- 13.Rehman SU, Amoaku WMK, Doran RM, et al. Randomized controlled clinical trial of beta irradiation as an adjunct to trabeculectomy in open angle glaucoma. Ophthalmology 2002;109:302–6. [DOI] [PubMed] [Google Scholar]
- 14.Crowston JG, Akbar AN, Constable PH, et al. Antimetabolite-induced apoptosis in Tenon’s capsule fibroblasts. Invest Ophthalmol Vis Sci 1998;39:449–54. [PubMed] [Google Scholar]
- 15.Ehrlich HP, Rajaratnam JBM. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound healing. Tiss Cell 1990;22:407–17. [DOI] [PubMed] [Google Scholar]
- 16.Orredson SU, Knighton DR, Scheuenstuhl H, et al. A quantitative in vitro study of fibroblast and endothelial cell migration in response to serum and wound fluid. J Surg Res 1983;35:249–58. [DOI] [PubMed] [Google Scholar]
- 17.Yanase A, Ueda M, Kaneda T, et al. Irradiation effects on wound contraction using a connective tissue model. Ann Plast Surg 1993;30:435–40. [DOI] [PubMed] [Google Scholar]
- 18.McDonald JA. In: Clark RAF, Henson PM, eds. The molecular and cellular biology of wound repair, 1 ed. New York: Plenum Press, 1988:405–35.
- 19.Miskulin M, Dalgleish R, Kluve-Beckerman B, et al. Human type III collagen gene expression is coordinately modulated with the type I collagen genes during fibroblast growth. Biochem 1986;25:1408–13. [DOI] [PubMed] [Google Scholar]
- 20.Rodemann HP, Peterson HP, Schwenke K, et al. Terminal differentiation of human fibroblasts is induced by radiation. Scan Microsc 1992;5:1135–43. [PubMed] [Google Scholar]