Giant cell arteritis (GCA) is a systemic vasculitis that affects large and medium sized arteries. Visual loss is one of the most devastating complications of GCA and usually occurs from occlusion of the posterior ciliary arteries (PCA) leading to anterior ischaemic optic neuropathy (AION). Visual loss can also occur from occlusion of other arteries that supply the visual pathway, such as the central retinal and ophthalmic arteries.
Corticosteroid therapy, given orally or intravenously, is the standard treatment for GCA associated visual loss.1 The optimal route of administration and dosage to prevent further visual loss are not known; however, most clinicians advocate higher doses in patients who already have experienced visual loss. Treatment with corticosteroids usually results in stabilisation of visual loss and some patients may have some degree of visual recovery.2,3 However, despite treatment with high dose intravenous corticosteroids, visual loss may progress.1 The reported use of adjunctive agents under these circumstances has been limited. We report a patient who had progressive visual loss while on high dose intravenous corticosteroids and who markedly improved after treatment with heparin.
Case report
An 85 year old man presented to his optometrist for a routine eye examination. His visual acuity was 20/40 both eyes and his optic discs were normal. Three weeks later (day 1), he lost vision in his right eye. His visual acuity was now 20/100 right eye and 20/40 left eye. On visual field testing with static perimetry he had an inferior altitudinal defect in the right eye and his visual field was normal in the left. His right optic disc was pale and oedematous and his left optic disc was normal. He was suspected of having GCA and was admitted for treatment with intravenous methylprednisolone (IVMP) 250 mg every 6 hours. On day 2, the visual field defect moved upward with involvement of fixation and his visual acuity was counting fingers. On day 3, the visual field defect increased further with a small rim of vision remaining superiorly. On day 4, his visual acuity was light perception (LP) and he was transferred to our institution.
On our initial examination (day 4), his visual acuity was LP right eye and 20/50 left eye. He had a right relative afferent pupillary defect (RAPD), pale optic disc oedema on the right, and a normal appearing optic disc on the left. The Westergren erythrocyte sedimentation rate (ESR) was 74 mm in the first hour. The IVMP was continued as before, and a temporal artery biopsy (TAB) was performed which was positive for GCA (fig 1).
Figure 1.
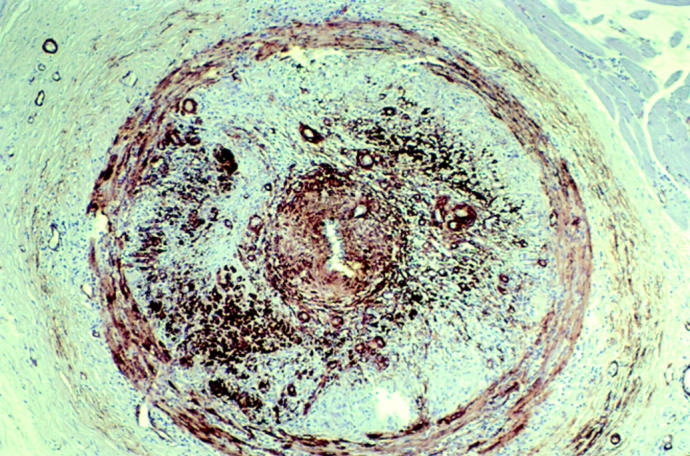
Photomicrograph of temporal artery biopsy histological section shows a marked proliferation of smooth muscle cells that can be seen as dark reddish/brown concentric rings in the thickened medial and intimal layers (stain, actin which is selective for smooth muscle cells, original magnification, ×250).
On day 5, his visual acuity decreased to no light perception (NLP) right eye and 20/80 left eye. His right pupil was amaurotic and his left pupil reacted sluggishly. Static perimetry now showed an inferior altitudinal defect in the left eye (fig 2A). The right optic disc appearance was unchanged but the superior portion of the left optic disc was now pale and oedematous. Posterior ciliary artery (PCA) blood flow could not be detected in either eye with orbital colour Doppler imaging (CDI) (table 1). Heparin (5000 units intravenous bolus) was started, and titrated to maintain the partial thromboplastin time (PTT) within a therapeutic range (46–70 seconds).
Figure 2.
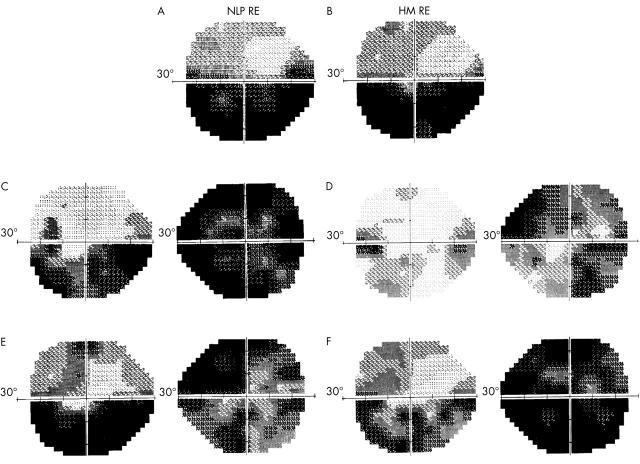
Serial automated static perimetry, (A) Day 5: mean deviation (MD) = −19.63 dB left eye, no light perception (NLP) right eye. (B) Day 6: MD = −17.99 dB LE, hand motion (HM) right eye. (C) Day 7: MD = −12.30 dB left eye, MD = −24.60 dB right eye. (D) Day 8: MD = −5.58 dB left eye, MD = −15.66 dB right eye. (E) Day 9: MD = −17.63 dB left eye, MD = −19.66 dB right eye. (F) Week 4: MD = −12.80 dB left eye, MD = −26.87 dB right eye.
Table 1.
Spectral analysis of orbital colour Doppler imaging
| Normal values11 (cm/s) | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 15 | |||||||
| RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | ||
| CRA systolic (cm/s) | 10.1+/−1.9 | 4.1 | 2.4 | 5.4 | 4.7 | 5.3 | 4.5 | 5.9 | 4.4 | 8.2 | 5.6 | 9.7 | 5.7 |
| CRA diastolic (cm/s) | 2.6+/−1.2 | 0.2 | 0.4 | 0.4 | 0.6 | 1.3 | 1.4 | 1.6 | 0.7 | 2.4 | 1.6 | 3.0 | 1.7 |
| CRA pulsatility index | 1.52 | 20.0 | 6.3 | 49.0 | 8.2 | 42.0 | 32.0 | 3.9 | 4.2 | 1.0 | 2.0 | 2.8 | 1.87 |
| PCA systolic (cm/s) | 12.4+/−4.2 | ||||||||||||
| Nasal | NR | NR | NR | NR | 4.2 | NR | 5.1 | NR | 7.7 | NR | 8.0 | NR | |
| Temporal | NR | NR | NR | NR | 3.1 | 7.2 | 4.8 | 9.1 | 7.4 | 9.4 | 7.4 | 5.0 | |
| PCA diastolic (cm/s) | 4.3+/−2.2 | ||||||||||||
| Nasal | NR | NR | NR | NR | 0.8 | NR | 0.4 | NR | 2.6 | NR | 2.3 | NR | |
| Temporal | NR | NR | NR | NR | 0.5 | 1.3 | 1.2 | 0.2 | 2.3 | 0.2 | 2.7 | 1.7 | |
| PCA pulsatility index | 1.09 | ||||||||||||
| Nasal | NR | NR | NR | NR | 1.8 | NR | 1.0 | NR | 1.3 | NR | 2.0 | NR | |
| Temporal | NR | NR | NR | NR | 7.7 | 3.0 | 3.1 | 3.9 | 1.7 | 2.2 | 1.9 | 3.0 | |
| OA systolic (cm/s) | 31.3+/−4.2 | 58.0 | 133.4 | 65.1 | 118.9 | 65.7 | 118.6 | 59.9 | 94.9 | 58.0 | 71.5 | 58.0 | 50.3 |
| OA diastolic (cm/s) | 8.3+/−3.9 | 19.3 | 31.9 | 16.9 | 37.7 | 21.3 | 16.9 | 19.3 | 23.7 | 23.2 | 11.6 | 20.3 | 10.3 |
| OA pulsatility index | 1.55 | 1.2 | 1.4 | 1.2 | 1.2 | 1.1 | 1.5 | 1.2 | 1.2 | 1.0 | 1.4 | 1.1 | 2.1 |
CRA = central retinal artery, PCA = posterior ciliary artery, OA = ophthalmic artery, NR = not recordable.
On day 6, his PTT was 74 seconds and his visual acuity improved to hand motion (HM) right eye and 20/70 left eye. His right pupil reacted sluggishly and the left pupil reacted more briskly with a right RAPD. Static perimetry was essentially unchanged (fig 2B) and there was no appreciable change in the optic disc appearance. The ocular blood flow characteristics with orbital CDI (fig 3A) were unchanged (table 1).
Figure 3.
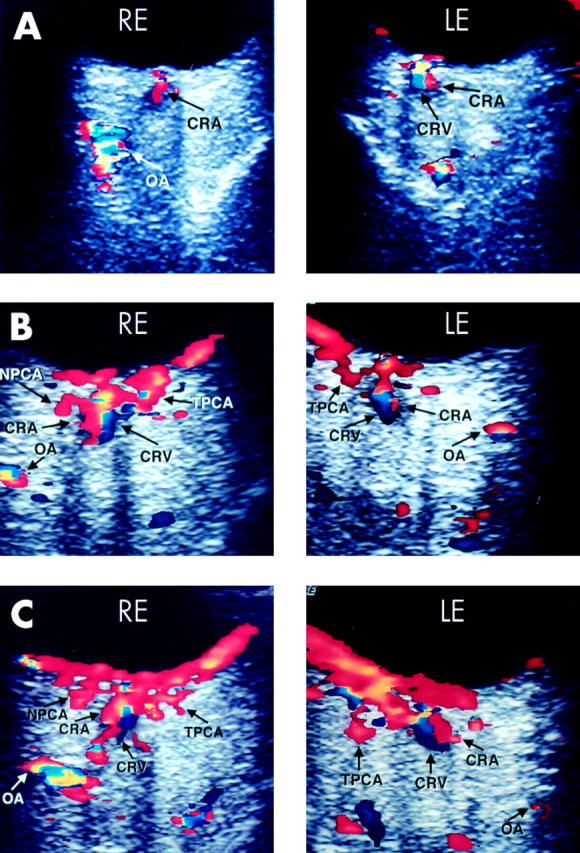
Serial orbital colour Doppler imaging. (A) Day 6: There is diminished blood flow through the ophthalmic artery (OA) in the right eye (OD) and central retinal artery (CRA) in both eyes. The blood flow through the posterior ciliary artery circulation is undetectable in both eyes. Blood flow through the central retinal vein (CRV) can be seen in the left eye (OS). (B) Day 9: 4 days after starting heparin, there is blood flow through the nasal posterior ciliary artery (NPCA) OD and through the temporal posterior ciliary arteries (TPCA) both eyes, with improved blood flow through the OA and CRA in both eyes. Blood flow through the CRV can be seen in both eyes. (C) Day 15: 6 days after discontinuation of heparin, the ocular blood flow remained stable.
On day 7, while continuing IVMP and heparin, his visual acuity improved to counting fingers (CF) at 1 foot right eye and 20/50 left eye. The pupillary examination was unchanged. Static perimetry was improved (fig 2C). Posterior ciliary artery blood flow was now detectable with orbital CDI (table 1). Warfarin (2.5 mg by mouth four times daily) was added and titrated to a therapeutic level.
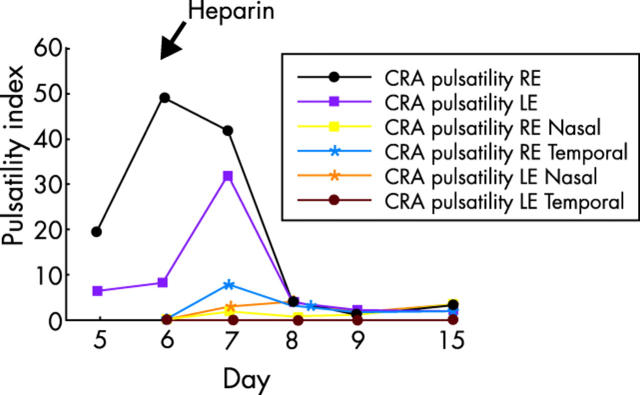
By day 8, his visual acuity had improved to 20/400 right eye and 20/50 left eye. Static perimetry was further improved (fig 2D). The ocular blood flow characteristics on orbital CDI likewise improved (table 1). The pulsatility index of the central retinal artery (CRA) was markedly decreased (fig 4). Oral corticosteroid therapy (prednisone 100 mg by mouth daily) was started and IVMP therapy was stopped.
Figure 4.
Graph of arterial pulsatility index. Blood flow through the posterior ciliary artery (PCA) was detectable on day 7 and central retinal artery (CRA) pulsatility index was nearly normal by day 8.
On day 9, the international ratio (INR) was therapeutic (between 2 and 3) and heparin was stopped. His visual acuity was 20/200 right eye and 20/70 left eye. Static perimetry was slightly worse in the right eye and the inferior altitudinal defect in the left eye was denser and closer to fixation (fig 2E). The ocular blood flow characteristics on orbital CDI (fig 3B) remained stable (table 1). The patient was discharged from the hospital with oral corticosteroid therapy (prednisone 100 mg by mouth daily) and warfarin.
At the 1 week follow up visit (day 15), his visual acuity improved to 20/80 right eye and 20/40 left eye. Static perimetry was unchanged. The ocular blood flow characteristics on orbital CDI (fig 3C) remained unchanged (table 1).
At the 4 week follow up visit while taking 100 mg of prednisone daily and warfarin, his visual acuity deteriorated to 20/400 right eye and 20/80 left eye. Static perimetry worsened in the right eye with diffuse loss and the inferior altitudinal defect left eye was less dense (fig 2F). The ocular blood flow characteristics on orbital CDI remained unchanged. At the 3 month follow up visit while still on the same medications, his visual acuity deteriorated to LP right eye and improved to 20/60 left eye. Static perimetry of the left eye was unchanged and the ocular blood flow characteristics on orbital CDI again remained stable bilaterally. His prednisone dosage was slowly tapered and the warfarin was discontinued.
Comment
Once visual loss occurs in GCA, the goals of treatment with corticosteroid therapy are to prevent further progression and to reverse the visual loss if possible. Table 2 reviews the treatment results of GCA with either oral or intravenous corticosteroid therapy in five large series of patients. After initiation of corticosteroid therapy, most patients experienced visual stabilisation, a small number had improvement of vision, and even fewer patients had a progression of visual loss.
Table 2.
Review of five large series of visual outcome in GCA treated with either oral or intravenous corticosteroid therapy
| Reference | Year | Total number of patients with GCA | Number of patients with visual loss | Number of patients (%) with visual stabilisation | Number of patients (%) with visual recovery | Number of patients (%) with progression of visual loss |
| Chan et al14 | 2001 | 73 | 73 | 43 (59) | 21 (29) | 9 (12) |
| Liozon et al7 | 2000 | 147 | 23 | 17 (74) | 5 (22) | 1 (4) |
| Gonzalez-Gay et al15 | 1998 | 239 | 34 | 22 (65) | 8 (23) | 4 (12) |
| Liu et al2 | 1993 | 45 | 41 | 20 (49) | 14 (34) | 7 (17) |
| Aiello et al3 | 1992 | 245 | 34 | 24 (70) | 5 (15) | 5 (15) |
The reason for progression of visual loss despite treatment with corticosteroid therapy is unknown. One possible reason is that the dose of corticosteroids was inadequate. Both the dose and route of administration of corticosteroids varied in the studies in table 2. Therefore, it is difficult to assess whether the treatment dose was adequate or if intravenous administration is superior to oral administration.
Most clinicians support the use of high dose intravenous corticosteroids for patients who have experienced visual loss from GCA. However, progression of visual loss has been described despite high dose IVMP.1,4–6
Another possible reason for the progression of visual loss despite treatment is that corticosteroid therapy alone is an inadequate treatment for some patients. A review of our patient’s TAB showed near total luminal stenosis by intimal hyperplasia with proliferation of actin stained smooth muscle cells in the arterial media and intimal layers (fig 1). Complete arterial occlusion can occur with progression of the hyperplastic process or may be the result of superimposed arterial thrombosis. Perhaps with advanced luminal stenosis, corticosteroids are not as effective as they are early in the inflammatory cascade and may not be able to prevent thrombosis.
We found one report of the use of heparin for visual loss in GCA.7 In a review of 174 patients with GCA, Liozon et al reported the use of heparin in most patients with permanent visual loss and some patients with threatening symptoms.7 However, the specific results of this treatment were not reported. The use of heparin has also been described for manifestations of GCA other than visual loss. Staunton et al described a 64 year old man with TAB proved GCA and clinical signs of an evolving vertebrobasilar stroke and ischaemic cerebellar lesion on MRI while on oral corticosteroid therapy.8 His symptoms improved following treatment with high dose intravenous dexamethasone and heparin. It has been postulated that systemic anticoagulation may be beneficial during the initial phase of steroid treatment because of recent evidence that anti-cardiolipin antibodies are present in a higher frequency in patients with GCA.9
Normally, heparin occurs complexed to histamine as a macromolecule in mast cells and its physiological role is unknown. Heparin has an immediate anticoagulation effect after intravenous administration. The coagulation process generates thrombin by two interrelated pathways, the extrinsic and intrinsic. Both pathways involve a cascade of enzymatic reactions that ultimately form thrombin. Thrombin catalyses the conversion of fibrinogen to fibrin that forms the matrix of a thrombus. Thrombin also activates clotting factor XIII that is necessary for stabilising the cross links of the fibrin molecules. If thrombin is not produced or production is impeded then coagulation is inhibited. Antithrombin III is an α-globulin that inhibits thrombin. Heparin indirectly binds to antithrombin III and forms a complex that more rapidly inhibits thrombin formation, thereby preventing coagulation and clot formation.
Heparin has also been shown to have other biochemical activities such as regulation of lipid metabolism, control of blood fluidity at the endothelial surface, control of cell attachment to various proteins in the extracellular matrix, binding with acidic and basic fibroblast growth factors, binding to interleukin 3 and granulocyte-macrophage colony stimulating factor, and inhibition of serotonin induced pulmonary artery smooth muscle cell hypertrophy.10 The mechanism by which heparin led to improvement of vision in our patient is not known and perhaps its therapeutic effect was unrelated to anticoagulation. None the less, serial orbital CDI showed improvement in ocular blood flow (table 1; fig 3A–C). Colour Doppler imaging is an ultrasonic imaging modality that combines B-mode ultrasonography with Doppler ultrasonography. When applied to the orbit, this imaging modality allows for the assessment of ocular blood flow and has been described in detail elsewhere.11,12 Blood flow towards the transducer is displayed as red and represents arterial flow and blood flow away from the transducer is displayed as blue and represents venous flow. Pulsed Doppler with spectral analysis can be used in conjunction with the CDI to accurately quantify the systolic and diastolic flow characteristics. The pulsatility index can be calculated and represents an assessment of vascular resistance to blood flow. A high pulsatility index indicates a high resistance and therefore reduced blood flow.
Initially, the systolic and diastolic pressures of the CRA were well below normal and the pulsatility index was markedly increased. The flow parameters of the PCA were undetectable. After heparinisation (day 5), flow was restored to the PCA circulation (day 7, table 1). The pulsatility index of the CRA also decreased (days 6–8, fig 4). The decrease in pulsatility index represents a decrease in blood flow resistance. The decrease in pulsatility index and restoration of blood flow to the PCA circulation both correspond to an improvement in visual acuity and visual field. The sequence of rapid improvement of orbital CDI haemodynamics, visual acuity, and visual field strongly suggest that the administration of heparin was responsible. Since this occurred rapidly, some of the biochemical activities of heparin, such as inhibition of smooth muscle cell hypertrophy and the others that act on a more chronic time line could not have caused improvement. A more likely candidate might be the control of blood fluidity at the endothelial surface.
In summary, the use of corticosteroid therapy alone for the treatment of GCA may have limited therapeutic success. The pathogenic mechanism of luminal stenosis in GCA is undergoing revision,13 and the emerging model offers possibilities for novel therapeutic intervention. Our patient had a remarkable improvement in visual acuity within 24 hours after starting heparin. Over the ensuing 2 days his visual field and ocular blood flow improved. The improvement with the addition of heparin may have been coincidental, and his vision may have recovered with IVMP alone. However, the continued decline in visual function, despite 3 days of IVMP, and his dramatic improvement after starting heparin, strongly suggests that heparin had a pivotal role in his recovery.
At the 3 month follow up visit, his vision in the right eye deteriorated to LP, even though his ocular blood flow characteristics were stable. We are uncertain about the reason for his decline in visual function, since at the time of the decline, he was maintained on oral corticosteroid and his serial ESR values were normal. None the less, we suggest that in patients who experience a progression of visual loss from GCA, despite IVMP therapy, may benefit acutely from the addition of heparin therapy.
Originally presented in part at the 28th Annual North American Neuro-Ophthalmology Society (NANOS) Meeting in Copper Mountain Resort, CO, USA, 9–14 February, 2002.
References
- 1.Cornblath WT, Eggenberger ER. Progressive visual loss from giant cell arteritis despite high-dose intravenous methylprednisolone. Ophthalmology 1997;104:854–8. [DOI] [PubMed] [Google Scholar]
- 2.Liu GT, Glaser JS, Schatz NJ, et al. Visual morbidity in giant cell arteritis. Clinical characteristics and prognosis for vision. Ophthalmology 1994;101:1779–85. [DOI] [PubMed] [Google Scholar]
- 3.Aiello PD, Trautmann JC, McPhee TJ, et al. Visual prognosis in giant cell arteritis. Ophthalmology 1993;100:550–5. [DOI] [PubMed] [Google Scholar]
- 4.Matzkin DC, Slamovits TL, Sachs R, et al. Visual recovery in two patients after intravenous methylprednisolone treatment of central retinal artery occlusion secondary to giant-cell arteritis. Ophthalmology 1992;99:68–71. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg DA, Savino PJ, Sergott RC, et al. Giant cell arteritis. Corticosteroids, temporal artery biopsy, and blindness. Arch Fam Med 1994;3:623–7. [DOI] [PubMed] [Google Scholar]
- 6.Slavin ML, Margolis AJ. Progressive anterior ischemic optic neuropathy due to giant cell arteritis despite high-dose intravenous corticosteroids. Arch Ophthalmol 1988;106:1167. [DOI] [PubMed] [Google Scholar]
- 7.Liozon E, Herrmann F, Ly K, et al. Risk factors for visual loss in giant cell (temporal) arteritis: a prospective study of 174 patients. Am J Med 2001;111:211–17. [DOI] [PubMed] [Google Scholar]
- 8.Staunton H, Stafford F, Leader M, et al. Deterioration of giant cell arteritis with corticosteroid therapy. Arch Neurol 2000;57:581–4. [DOI] [PubMed] [Google Scholar]
- 9.Levesque H, Cailleux N, Courtois H. On the necessity of anticoagulant therapy during the initial phase of steroid treatment in giant cell arteritis. Eur J Med 1993;2:186–7. [PubMed] [Google Scholar]
- 10.Garg HG, Thompson BT, Hales CA. Structural determinants of antiproliferative activity of heparin on pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2000;279:L779–89. [DOI] [PubMed] [Google Scholar]
- 11.Aburn NS, Sergott RC. Orbital colour Doppler imaging. Eye 1993;7(Pt 5):639–47. [DOI] [PubMed] [Google Scholar]
- 12.Lieb WE, Flaharty PM, Ho A, et al. Color Doppler imaging of the eye and orbit. A synopsis of a 400 case experience. Acta Ophthalmol Suppl 1992:50–4. [DOI] [PubMed]
- 13.Weyand CM. The Dunlop-Dottridge Lecture: The pathogenesis of giant cell arteritis. J Rheumatol 2000;27:517–22. [PubMed] [Google Scholar]
- 14.Chan CC, Paine M, O’Day J. Steroid management in giant cell arteritis. Br J Ophthalmol 2001;85:1061–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Gay MA, Blanco R, Rodriguez-Valverde V, et al. Permanent visual loss and cerebrovascular accidents in giant cell arteritis: predictors and response to treatment. Arthritis Rheum 1998;41:1497–504. [DOI] [PubMed] [Google Scholar]