Abstract
Background/aim: The Heidelberg retina flowmeter (HRF) is designed to measure retinal capillary blood flow. Previous studies however showed weak reproducibility of data. The intraindividual reproducibility of circadian HRF measurements was examined in healthy subjects in three locations of the retina.
Methods: 36 healthy volunteers (27.3 (SD 4.3) years) were examined by HRF seven times a day (t0–t6). Using a default window of 10×10 pixels, three consecutive measurements were performed in three precise focusing planes: superficial, intermediate and deep layer, peripapillary retina, neuroretinal rim and cup, respectively. Images of identical tissue locations identified by capillary landmarks of each layer were selected to quantify the retinal microcirculation of each volunteer. Means and standard deviations of all flow results of a given subject were calculated, at t0–t6 and the coefficients of variation as a measure of reproducibility.
Results: The coefficients of variation ranged between 8.4% and 41.0% in the superficial layer (mean 19.8% (SD 8.4%)), 10.6%, and 43.0% in the intermediate layer (mean 24.0% (SD 8.4%)), and 9.9% and 84.0% (mean 29.6% (SD 15.8%)) in the deep layer.
Conclusions: These data show the best reproducibility of measurements in the superficial layer followed by the intermediate and the deep layer. Clinically, this is an unsatisfactory intraindividual reproducibility of flow values in each studied layer.
Keywords: blood flow, Heidelberg retina flowmeter, glaucoma, laser Doppler flowmetry, neuroretinal rim
The optic nerve head (ONH) is a central structure to survey clinical alteration in chronic eye diseases like glaucoma. Glaucomatous damage and atrophy may be influenced by vascular changes,1–5 but the pathogenesis of visual field loss remains unknown. Additionally, glaucomatous changes have been associated with local and systemic factors, which may influence the optic nerve circulation—for example, arterial hypertension and hypotension, altered autoregulation of ocular circulation in the ONH, and nocturnal hypotension. Consequently there is interest in finding an accurate and precise diagnostic method for microcirculatory assessment in the ONH. The Heidelberg retina flowmeter (HRF, Heidelberg Engineering GmbH, Dossenheim, Germany) measures retinal capillary blood flow by combining the principles of scanning laser Doppler flowmetry and confocal scanning laser techniques.6 The vascular theory in glaucoma suggests blood flow anomalies may influence disease progression. Our goal was to evaluate the ability of the HRF to detect changes in vascular systems at different locations in and around the ONH, performing seven consecutive measurements in one day. Previous studies, however, have shown poor reproducibility. We examined intraindividual reproducibility of HRF measurements in healthy subjects in three defined measurement locations within separate tissue layers.
METHODS
Thirty six eyes of 36 healthy volunteers (19 women, 17 men, mean age 27.9 (SD 4.4) years, age range 19.2–39.1 years) were examined by HRF (Operation software release 1.02) at baseline (t0) and six consecutive measurements after 2, 3, 4, 6, 8, and 10 hours (t1–t6).
All procedures conformed to the tenets of the Declaration of Helsinki, and were approved by the institutional review board, with subjects giving informed consent.
Volunteers were normotensive, had no intake of drugs influencing haemorheology (10 days preceding the examination), no history of ocular disease, corrected visual acuity 20/30 or better, intraocular pressure (IOP) <22 mm Hg, refractive error between –6.00 and +1.25 dioptres (mean −1.13 dioptres (SD 1.46)) and astigmatism <1.50 dioptres. This ocular anatomy allowed HRF measurements of the ONH perfusion with at least one window placed at the bottom of the disc excavation.
HRF is a non-invasive method for acquiring a high definition map of perfused retinal vessels and the ONH using confocal technique, often described in detail.5,7–10 The light source of the HRF is a diode laser at 780 nm wavelength and power between 100 µW and 200 µW. The laser is focused on the desired axial plane, and an area of 10°×2.5° is scanned. Two green lines on the examiner’s screen mark the boundaries of the measurement field. The laser is centred on the tissue of interest, adjusting focus to produce ideal brightness. Sensitivity is set to optimise the brightest pixels in light yellow colour, avoiding white or brown pixels. Picture resolution is 256×64 pixels. Each of the 64 horizontal lines is scanned 128 times with a line repetition rate of 4 kHz. Final data sets contain 128 measurements at each pixel.
By interpolation, these data are converted into a continuous wave function over time for each pixel. A spectrum analysis from the data of each location is performed using a FFT (fast Fourier transformation) algorithm and the power spectrum of the Doppler shift of each retinal point is calculated, computing to blood flow parameters. Image resolution (1 pixel) equals 10 µm ×10 µm for an imaged retinal area of 10×2.5 degrees. The standard measurement window is 10×10 pixels, representing a 100×100×400 µm volume of retinal tissue. Flow, volume, and velocity parameters are measured in arbitrary units (AU). All three parameters are calculated from the same basis using different formulas.
The HRF images were acquired using a 10×2.5 degree measurement field of 256 points ×64 lines. One experienced examiner acquired all images. One image was centralised on the papilla at each measurement (t0–t6). Measurements were performed in three focusing planes, superficial, intermediate and deep layers, peripapillary, neuroretinal rim, and cup respectively (fig 1). All HRF recordings were obtained through undilated pupils (more than 3 mm diameter). Only images without blinking or eye movements were considered for analysis. To ensure a reliable focus setting it was adjusted adding 0.5 dioptres for each step towards deeper layers, sensitivity was changed to provide best possible tissue reflectivity. Signs of over-illumination, white pixels, and under-illumination, dark brown pixels, were avoided. To avoid errors resulting from accommodative changes between single images a fixation point was utilised at 2.5 metres.
Figure 1.
Positions of the default measurement window of 10×10 pixels shown on a schematic drawing of the optic nerve head, according to the three focusing planes: superficial layer (□), intermediate layer (○), and deep layer (▵).
The sample box was placed on an area free from motion artefacts and major vessels with a default window of 10×10 pixels, on each focusing plane. Images of identical tissue locations identified by capillary landmarks of each layer were selected to quantify retinal microcirculation (fig 2A–C).
Figure 2.
Positions of the default measurement window of 10×10 pixels shown on the HRF image according to the three focusing planes: superficial layer (A), intermediate layer (B), and deep layer (C).
Statistics
Means and standard deviations were calculated of all flow results of a given subject at t0–t6 and the coefficients of variation as a measure of reproducibility. A variance component estimation procedure was also performed.
The Friedman test (two way nonparametric analysis of variance (ANOVA)) was used to compare flow and DC values among three groups. Between two groups, the Wilcoxon signed rank test with multiple comparison procedure was applied. The p values <0.05 and 0.016 for Friedman and Wilcoxon signed rank test, respectively, were regarded as statistically significant.
RESULTS
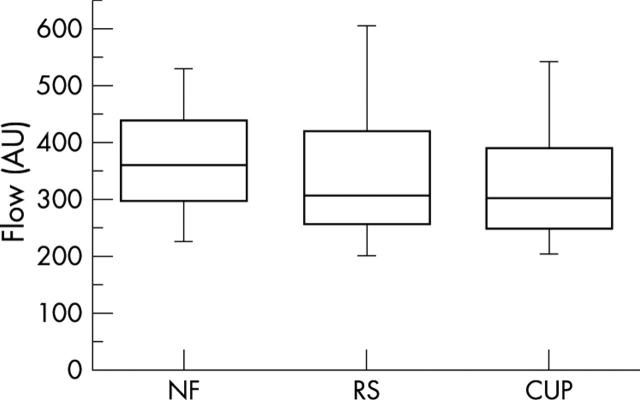
Table 1 shows means and standard deviations of flow values in arbitrary units (AU), measured in three different tissue layers for each subject. The number of examined pixels was 100 for every measurement frame and layer, defined by the 10×10 pixel default window. For the superficial layer (NF = peripapillary retina), flow values ranged between 203.12 and 579.93 AU (mean 371.74 AU (SD 106.22)). In the intermediate layer (RS = neuroretinal rim), we calculated flow values between 143.8 and 918.58 AU (mean 363.97 AU (SD 171.46)). Finally, flow values in the deep layer (Cup) ranged between 125.5 and 920.39 AU (mean 342.43 AU (SD 146.07)). Mean flow values for the superficial layer were higher than those of the intermediate and deep layers, without statistically significant differences among the subgroups (fig 3).
Table 1.
Mean flow values (mean) and standard deviation (SD) for 36 subjects for measurements in three focusing planes: superficial layer, intermediate layer, and deep layer
| Subject | Superficial layer | Intermediate layer | Deep layer | |||
| Mean | SD | Mean | SD | Mean | SD | |
| 1 | 431.665 | 49.593 | 273.727 | 65.694 | 319.951 | 61.799 |
| 2 | 579.931 | 76.571 | 489.294 | 97.057 | 548.606 | 146.740 |
| 3 | 495.267 | 70.226 | 266.656 | 88.205 | 408.812 | 74.368 |
| 4 | 551.801 | 47.403 | 369.245 | 62.385 | 398.058 | 77.814 |
| 5 | 317.705 | 83.137 | 259.939 | 61.719 | 249.811 | 48.245 |
| 6 | 444.686 | 50.385 | 260.441 | 56.152 | 283.274 | 72.076 |
| 7 | 365.845 | 73.702 | 143.798 | 54.011 | 238.767 | 62.389 |
| 8 | 448.474 | 102.522 | 521.610 | 224.044 | 239.987 | 201.481 |
| 9 | 408.587 | 49.245 | 251.592 | 34.837 | 362.830 | 35.803 |
| 10 | 319.041 | 26.915 | 192.191 | 36.938 | 363.769 | 58.673 |
| 11 | 421.488 | 84.286 | 705.313 | 106.848 | 335.566 | 73.359 |
| 12 | 361.097 | 40.009 | 231.572 | 38.625 | 356.013 | 87.465 |
| 13 | 368.851 | 50.945 | 486.373 | 59.200 | 920.394 | 409.432 |
| 14 | 353.461 | 48.292 | 261.331 | 96.969 | 271.658 | 87.923 |
| 15 | 534.847 | 156.300 | 918.584 | 127.316 | 201.348 | 99.303 |
| 16 | 559.113 | 97.549 | 550.526 | 157.331 | 330.911 | 92.626 |
| 17 | 225.779 | 45.099 | 300.484 | 111.449 | 296.892 | 73.513 |
| 18 | 218.403 | 76.887 | 422.650 | 94.072 | 199.765 | 125.778 |
| 19 | 286.269 | 80.886 | 173.949 | 43.998 | 209.106 | 97.741 |
| 20 | 234.444 | 96.205 | 210.587 | 42.636 | 125.501 | 62.265 |
| 21 | 221.251 | 38.678 | 347.613 | 70.730 | 180.032 | 77.379 |
| 22 | 491.439 | 57.324 | 200.957 | 37.254 | 273.700 | 45.695 |
| 23 | 301.929 | 62.803 | 288.169 | 55.404 | 306.111 | 140.237 |
| 24 | 307.611 | 57.794 | 397.641 | 140.909 | 379.466 | 122.314 |
| 25 | 404.836 | 51.052 | 317.048 | 85.011 | 271.037 | 33.639 |
| 26 | 499.356 | 73.260 | 327.861 | 80.360 | 391.198 | 89.483 |
| 27 | 329.772 | 46.215 | 418.269 | 44.385 | 416.109 | 57.177 |
| 28 | 351.268 | 66.901 | 730.769 | 182.961 | 551.106 | 137.144 |
| 29 | 419.113 | 93.756 | 300.894 | 67.338 | 395.326 | 85.134 |
| 30 | 291.554 | 44.735 | 358.399 | 80.887 | 304.196 | 68.491 |
| 31 | 405.084 | 130.856 | 419.951 | 109.734 | 528.797 | 108.927 |
| 32 | 225.896 | 48.231 | 615.381 | 135.617 | 303.081 | 75.060 |
| 33 | 203.120 | 69.763 | 210.561 | 63.085 | 244.279 | 80.313 |
| 34 | 437.096 | 72.554 | 343.531 | 67.662 | 592.404 | 60.798 |
| 35 | 309.527 | 93.043 | 300.100 | 46.292 | 249.028 | 61.265 |
| 36 | 256.946 | 84.814 | 235.998 | 100.694 | 280.536 | 133.413 |
Figure 3.
Box plots of total flow values for 36 subjects for measurements in three focusing planes: superficial layer (NF), intermediate layer (RS), deep layer (Cup).
The mean DC values, reflecting the tissue brightness, were 136.46 (SD 34.01) for the superficial layer, 53.65 (SD 29.68) intermediate layer, and 159.36 (SD 45) deep layer. Differences were statistically significant between superficial and intermediate layers (Friedman test: p<0.0001, Wilcoxon rank signed test: p<0.0001), between superficial and deep layer (Friedman test: p<0.0001, Wilcoxon rank signed test: p = 0.0143), and between intermediate and deep layer (Friedman test: p<0.0001, Wilcoxon rank signed test: p<0.0001) (fig 4).
Figure 4.
Box plots of total DC values for 36 subjects for measurements in three focusing planes: superficial layer (NF), intermediate layer (RS), deep layer (Cup). *Significant difference between NF and RS (p<0.0001, Wilcoxon signed rank test); †significant difference between NF and Cup (p = 0.0143, Wilcoxon signed rank test); ▴significant difference between RS and Cup (p = 0.0143, Wilcoxon signed rank test).
Considering the DC component between 70 and 200 as defining the required brightness for a high quality image, the percentage of unacceptably illuminated tissue was 2.8% for the superficial layer, 22.2% for the intermediate layer, and 75.0% for the deep layer (fig 5).
Figure 5.
Histogram of bad DC percentage (DC <70 and >200) for 36 subjects for measurements in three focusing planes: superficial layer (NF), intermediate layer (RS), deep layer (Cup).
The means for the remaining acceptable DC values of all three different layers were 138.82 (SD 31.43) superficial layer, 95.69 (SD 24.32) intermediate layer, and 149.69 (SD 32.79) deep layer. Mean DC value of the superficial layer was significantly higher than that of the intermediate layer (Friedman test: p = 0.0098, Wilcoxon rank signed test: p = 0.0077) (fig 6).
Figure 6.
Box plots of DC values for 36 subjects for measurements in three focusing planes: superficial layer (NF), intermediate layer (RS), deep layer (Cup), leaving out cases with bad DC values (defined as DC<70 and >200). *Significant difference between NF and RS (p = 0.0077, Wilcoxon signed rank test).
For acceptable DC values (>70 and <200), the mean flow values were 138.82 AU (SD 31.43) in the superficial layer, 95.69 AU (SD 24.32) in the intermediate layer, and 149.68 AU (SD 32.79) in the deep layer, without statistically significant differences between the subgroups (fig 7).
Figure 7.
Flow value box plots of for measurements in three focusing planes: superficial layer (NF), intermediate layer (RS), deep layer (Cup), leaving out cases with bad DC values (defined as DC<70 and >200).
Additionally, the reproducibility coefficients of variation were calculated and shown as box plots in figure 8. The coefficients of variation ranged between 8.4% and 41.0% in the superficial layer (mean 19.8 % (SD 8.4)), between 10.6% and 43.0% in the intermediate layer (mean 24.0 % (SD 8.4)), and between 9.9% and 84.0% (mean 29.6 % (SD 15.8)) in the deep layer. Table 2 shows a listing of the coefficients of variation of each individual subject.
Figure 8.
Coefficient of variation for 36 subjects for measurements in three focusing planes: superficial layer (NF), intermediate layer (RS), deep layer (Cup). *Significant difference between NF and Cup (p<0.0001, Wilcoxon signed rank test).
Table 2.
Coefficient of variation for 36 subjects for measurements in three focusing planes: superficial layer (NF), intermediate layer (RS), deep layer (Cup)
| Subject | NF | RS | Cup |
| 1 | 11.5 | 24.0 | 19.3 |
| 2 | 13.2 | 19.8 | 26.7 |
| 3 | 14.2 | 33.1 | 18.2 |
| 4 | 8.6 | 16.9 | 19.5 |
| 5 | 26.2 | 23.7 | 19.3 |
| 6 | 11.3 | 21.6 | 25.4 |
| 7 | 20.1 | 37.6 | 26.1 |
| 8 | 22.9 | 43.0 | 84.0 |
| 9 | 12.1 | 13.8 | 9.9 |
| 10 | 8.4 | 19.2 | 16.1 |
| 11 | 20.0 | 15.1 | 21.9 |
| 12 | 11.1 | 16.7 | 24.6 |
| 13 | 13.8 | 12.2 | 44.5 |
| 14 | 13.7 | 37.1 | 32.4 |
| 15 | 29.2 | 13.9 | 49.3 |
| 16 | 17.4 | 28.6 | 28.0 |
| 17 | 20.0 | 37.1 | 24.8 |
| 18 | 35.0 | 22.3 | 63.0 |
| 19 | 28.3 | 25.3 | 46.7 |
| 20 | 41.0 | 20.2 | 49.6 |
| 21 | 17.5 | 20.3 | 43.0 |
| 22 | 11.7 | 18.5 | 16.7 |
| 23 | 20.8 | 19.2 | 45.8 |
| 24 | 18.8 | 35.4 | 32.2 |
| 25 | 12.6 | 26.8 | 12.4 |
| 26 | 14.7 | 24.5 | 22.9 |
| 27 | 14.0 | 10.6 | 13.7 |
| 28 | 19.0 | 25.0 | 24.9 |
| 29 | 22.4 | 22.4 | 21.5 |
| 30 | 15.3 | 22.6 | 22.5 |
| 31 | 32.3 | 26.1 | 20.6 |
| 32 | 21.4 | 22.0 | 24.8 |
| 33 | 34.3 | 30.0 | 32.9 |
| 34 | 16.6 | 19.7 | 10.3 |
| 35 | 30.1 | 15.4 | 24.6 |
| 36 | 33.0 | 42.7 | 47.6 |
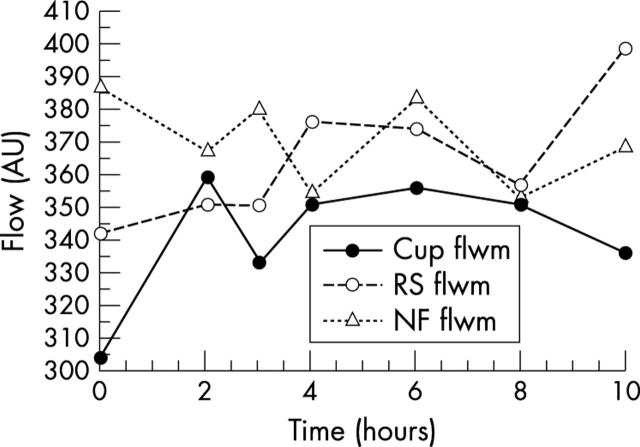
According to the variance component estimation procedure, the highest influencing factors were in decreasing order; individual subject, measurement location, and refractive error. Circadian flow measurements showed no statistically significant differences between individual measurement points of time. Figure 9 shows circadian mean flow values for all three flow locations. Standard deviations (SD) of flow values at all measurement points are presented in figure 10. There were smaller standard deviations in the superficial layer for all circadian measurements.
Figure 9.
Line diagram of mean flow values for measurements in three focusing planes: superficial layer (NF), intermediate layer (RS), deep layer (Cup), for all measured flow values.
Figure 10.
Line diagram of standard deviations for flow measurements in three focusing planes: superficial layer (NF), intermediate layer (RS), deep layer (Cup), for all measured flow values.
DISCUSSION
Glaucomatous optic disc lesions, resulting in optic nerve atrophy, may be associated with disturbances of ocular microcirculation.5,11
HRF should measure blood flow in retinal tissue. Usually, the peripapillary retina is focused in an image frame with the optic disc at its centre. Many studies image the ONH and peripapillary retina simultaneously. Some groups focus only on peripapillary retina, excluding other posterior located structures as they are not in the plane.12,13
We aimed to evaluate intraindividual circadian reproducibility of HRF blood flow measurements and investigate if the most common technique using the default settings of the commercially available software is suitable for clinical use.
Measurements were performed on three separate layers, the superficial, intermediate, and deep layers, changing the focal plane. Deeper layers were investigated because the neuroretinal rim, and cup are tissues where blood flow alterations can occur, possibly including ciliary source ONH vessels.
Previous studies showed coefficients of reproducibility between 0.7 and 0.95 for flow measurements using the default measurement window.9,14–17 In contrast with high reproducibility in short term comparisons, long term studies provide higher variability of flow results. Long term reproducibility may be increased by analysis of the entire image.7 Additionally, factors including the cardiac cycle and pulsatility of retinal flow should be considered in studies using the HRF.18
We showed best reproducibility in the superficial layer followed by the intermediate and deep layer.
HRF flow measurements have experienced problems of unstable baselines. In one study, 15 healthy volunteers were examined weekly for 1 month.19 The results showed that either HRF measurements were not stable over 1 month or that retinal capillary blood flow varied widely. Another investigator used calculated flow changes as a percentage of baseline in order to reduce the influence of an unstable baseline.20 Another study found good reproducibility of repeated interobserver measurements of the retina and lamina cribrosa with the default measurement window of 10×10 pixels, but poor reproducibility in the neuroretinal rim area.17 They also described coefficients of variation from consecutive intraindividual measurements ranging from 3.43% to 71.04%, depending on measurement location and measurement window size. For flow measurements and a default window size of 10×10 pixels they calculated mean coefficients of variation of 21.52% (SD 10.51%) for retinal location, 25.22% (SD 16.49%) for neuroretinal rim, and 23.11% (SD 15.82%) for lamina cribrosa.17 We found higher coefficients of variability in the lamina cribrosa than the neuroretinal rim.
Assessing circadian intraindividual flow measurements, there were no statistically significant differences between individual measurement points of time for all three locations (fig 9). This reflects high circadian reproducibility for each measurement layer. According to the SD of flow values at all measurement points during the day, the smallest standard deviations for circadian measurements were in the superficial layer (fig 10); in comparison, measurements in the intermediate and deep layer showed higher SD. This indicates weaker reproducibility of intraindividual measurements in these two layers for every measurement.
We also evaluated blood flow values depending on location of the measurement frame. The highest flow values were in the superficial layer, followed by the intermediate and deep layer. These results are similar to the literature.21 Other examiners found higher flow values in normals in the neuroretinal rim area than the juxtapapillary retina, but without quantified change of focal plane according to location, only depending on quality adjustments under monitor image control.22 However, these flow values depend on the brightness of the measured pixels. Brightness is the only help for adjustment during image acquisition. Depending on brightness, flow values can vary widely, like other researchers showed in vitro and in vivo.7,10,23 Brightness or reflectivity is described in HRF by the DC parameter. We also evaluated DC values in each of the layers. Figure 4 indicates statistically significant differences between the layers, with lowest mean DC values for the intermediate layer, higher values for the superficial layer, and highest values for the deep layer. HRF uses a noise correction algorithm depending on the image brightness, so differences in flow values can be expected if different DC values ranges are used. Using a range from 70–200 for DC values, often described as acceptable illumination for HRF images, considerable amount of data was found in the intermediate layer (75%) not complying with the requirements for good image quality. Interestingly, many descriptions in other studies use the statement that images in focus or best available quality were taken, without presenting the image related DC values.14,17,24 Figure 5 shows the distribution of bad DC values among the layers in this study, with lowest poor values for the superficial layer (2.8%). Omitting measurements corresponding to unacceptable DC values, mean DC values increase considerably in the intermediate layer and decrease slightly in the deep layer, remaining nearly the same in the superficial layer (fig 6). Flow values in all three layers clearly decrease (fig 7). Almost stable values for the superficial layer might be attributed to optimum focusing being achieved during image acquisition. Optimum focus is achieved according to tissue structure clarity, which results in most extensive surface of optical image control. With each deeper layer, the tissues of interest are smaller, resulting in more difficult sensitivity adjustment. We used clearly defined steps in refractive correction to compensate for increasing depth from superficial to deep layer, removing one additional error source during image acquisition.
According to variance component estimation analysis, factors influencing flow measurements in decreasing order are individual subject, measurement location, and subject’s refractive error. Certainly, each subject provides different flow results, depending on individual vascular anatomy and related perfusion, which may account for substantial influence on measurements.
Another factor affecting blood flow measurements with HRF is location dependence. Although the 10×10 pixel measurement has been described as optimal in reproducible measurements of optic nerve head blood flow,25 the small default measurement frame provides localised results and small location changes lead to important differences in values.
One study found that measurements in a 100×100×400 µm volume of peripapillary retinal tissue provide results independent of laser beam incidence angle to fundus. However, camera distance from the eye influences measurement of blood flow.7 Hence, we tried to avoid location dependent measurement errors by centring the ONH and adjusting the measurement window exactly for each separate image of an individual eye according to capillary and vessel landmarks, which is easier in the superficial layer than in the intermediate and deeper layers. Slight movement of localisation, even if only one pixel, can alter blood flow values. This fact also influences comparability of longitudinal intraindividual blood flow measurements, as in glaucoma follow up measurements.
Refractive errors have not been evaluated yet in relation to HRF measurements, thus the amount of influence remains speculative, although we found refraction to be the third influencing factor in our data.
In the commercially available HRF software, there is no method for exact transposition of the measurement window from one image to another in order to avoid location dependent errors. Therefore, examiners can also expect difficulties in positioning the default measurement window of 10×10 pixels on the neuroretinal rim of a glaucoma patient because of thinning of the tissue in disease progression indicating methods of analysis, like pointwise, using smaller measurement windows, more appropriate.26
In conclusion, there are several factors influencing blood flow measurements with HRF, including image brightness and location. Efforts are already under way to improve image quality acquisition by focusing on the required brightness in the tissue achieving proper illumination and consequently more reliable blood flow measurements.7,10,27,28 Concurrently, location dependence of measurements can be reduced, by using accurate tissue area definitions.27
Additionally, there are new software packages developed for HRF, containing various analysing capabilities, promising more accurate, reliable data.9,27,29
The above mentioned coefficients of variability in our study showed unsatisfactory reproducibility of flow values in each studied layer. In conclusion, the flow values in all three layers are measurement window location dependent. Our results found good circadian intraindividual reproducibility for all three measurement layers.
The standard release software limits the application for clinical purposes. Future releases should consider the limitations to avoid further sources of error and improve the reproducibility of measurements.
REFERENCES
- 1.Quigley HA, Hohman RM, Sanchez R, et al. Optic nerve head blood flow in chronic experimental glaucoma. Arch Ophthalmol 1985;103:956–62. [DOI] [PubMed] [Google Scholar]
- 2.Sossi N, Anderson DR. Effect of elevated intraocular pressure on blood flow. Occurrence in cat optic nerve head studied with iodoantipyrine I 125. Arch Ophthalmol 1983;101:98–101. [DOI] [PubMed] [Google Scholar]
- 3.Nanba K, Schwartz B. Nerve fiber layer and optic disc fluorescein defects in glaucoma and ocular hypertension. Ophthalmology 1988;95:1227–33. [DOI] [PubMed] [Google Scholar]
- 4.Michelson G, Groh MJ, Langhans M. Perfusion of the juxtapapillary retina and optic nerve head in acute ocular hypertension. Ger J Ophthalmol 1996;5:315–21. [PubMed] [Google Scholar]
- 5.Michelson G, Schmauss B, Langhans MJ, et al. Principle, validity, and reliability of scanning laser Doppler flowmetry. J Glaucoma 1996;5:99–105. [PubMed] [Google Scholar]
- 6.Riva CE, Harino S, Petrig BL, et al. Laser Doppler flowmetry in the optic nerve. Exp Eye Res 1992;55:499–506. [DOI] [PubMed] [Google Scholar]
- 7.Kagemann L, Harris A, Chung HS, et al. Heidelberg retinal flowmetry: factors affecting blood flow measurement. Br J Ophthalmol 1998;82:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollo G, Greve EL, van den Berg TJ, et al. Evaluation of the peripapillary circulation in healthy and glaucoma eyes with scanning laser Doppler flowmetry. Int Ophthalmol 97 1996;20:71–7. [DOI] [PubMed] [Google Scholar]
- 9.Michelson G, Welzenbach J, Pal I, et al. Automatic full field analysis of perfusion images gained by scanning laser Doppler flowmetry. Br J Ophthalmol 1998;82:1294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsang AC, Harris A, Kagemann L, et al. Brightness alters Heidelberg retinal flowmeter measurements in an in vitro model. Invest Ophthalmol Vis Sci 1999;40:795–9. [PubMed] [Google Scholar]
- 11.Michelson G, Groh MJ, Groh ME, et al. Advanced primary open-angle glaucoma is associated with decreased ophthalmic artery blood-flow velocity. Ger J Ophthalmol 1995;4:21–4. [PubMed] [Google Scholar]
- 12.Chung HS, Harris A, Kagemann L, et al. Peripapillary retinal blood flow in normal tension glaucoma. Br J Ophthalmol 1999;83:466–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung HS, Harris A, Halter PJ, et al. Regional differences in retinal vascular reactivity. Invest Ophthalmol Vis Sc 1999;40:2448–53. [PubMed] [Google Scholar]
- 14.Bohdanecka Z, Orgul S, Prunte C, et al. Influence of acquisition parameters on hemodynamic measurements with the Heidelberg retina flowmeter at the optic disc. J Glaucoma 1998;7:151–7. [PubMed] [Google Scholar]
- 15.Chauhan BC, Smith FM. Confocal scanning laser Doppler flowmetry: experiments in a model flow system. J Glaucoma 1997;6:237–45. [PubMed] [Google Scholar]
- 16.Michelson G, Schmauss B. Two dimensional mapping of the perfusion of the retina and optic nerve head. Br J Ophthalmol 1995;79:1126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolela MT, Hnik P, Schulzer M, et al. Reproducibility of retinal and optic nerve head blood flow measurements with scanning laser Doppler flowmetry. J Glaucoma 1997;6:157–64. [PubMed] [Google Scholar]
- 18.Michelson G, Harazny J. Relationship between ocular pulse pressures and retinal vessel velocities. Ophthalmology 1997;104:664–71. [DOI] [PubMed] [Google Scholar]
- 19.Martin B, Kagemann L, Buck S, et al. Variations in normal retinal capillary blood flow over one month as measured by Heidelberg retina flowmetry. Invest Ophthalmol Vis Sci 1997;38:S1049. [Google Scholar]
- 20.Strenn K, Matulla B, Wolzt M, et al. Reversal of endothelin-1-induced ocular hemodynamic effects by low-dose nifedipine in humans. Clin Pharmacol Ther 1998;63:54–63. [DOI] [PubMed] [Google Scholar]
- 21.Griesser SM, Lietz A, Orgul S, et al. Heidelberg retina flowmeter parameters at the papilla in healthy subjects. Eur J Ophthalmol 1999;9:32–6. [DOI] [PubMed] [Google Scholar]
- 22.Michelson G, Langhans MJ, Groh MJ. Perfusion of the juxtapapillary retina and the neuroretinal rim area in primary open angle glaucoma. J Glaucoma 1996;5:91–8. [PubMed] [Google Scholar]
- 23.Hosking SL, Embleton S, Kagemann L, et al. Detector sensitivity influences blood flow sampling in scanning laser Doppler flowmetry. Graefes Arch Clin Exp Ophthalmol 2001;239:407–10. [DOI] [PubMed] [Google Scholar]
- 24.Hollo G, van den Berg TJ, Greve EL. Scanning laser Doppler flowmetry in glaucoma. Int Ophthalmol 1996;20:63–70. [DOI] [PubMed] [Google Scholar]
- 25.Mizuki K, Yamazaki Y. Measurement of blood flow in the optic nerve head in glaucoma eyes using Heidelberg retina flowmeter. Jap J Clin Ophthalmol 1999;53:649–52. [Google Scholar]
- 26.Harris A, Kagemann L, Evans DW, et al. new method for evaluating ocular blood flow in glaucoma: pointwise flow analysis of HRF-imagesAssessment of human ocular hemodynamics. Invest Ophthalmol Vis Sci 1997;38:439. [Google Scholar]
- 27.Jonescu-Cuypers CP, Chung HS, Kagemann L, et al. New neuroretinal rim blood flow evaluation method combining Heidelberg retina flowmetry and tomography. Br J Ophthalmol 2001;85:304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kagemann L, Harris A, Chung H, et al. Photodetector sensitivity level and heidelberg retina flowmeter measurements in humans. Invest Ophthalmol Vis Sci 2001;42:354–7. [PubMed] [Google Scholar]
- 29.Iester M, Altieri M, Michelson G, et al. Intraobserver reproducibility of a two-dimensional mapping of the optic nerve head perfusion. J Glaucoma 2002;11:488–92. [DOI] [PubMed] [Google Scholar]