Abstract
Background: To investigate the levels of nitric oxide (NO) markers in plasma and aqueous humour of patients with primary open angle glaucoma (POAG) and their relation to ocular perfusion pressure.
Methods: Cyclic guanosine monophosphate (cGMP) and nitrite (NO2 −) were determined in plasma and aqueous humour of 38 patients with POAG and 46 controls. Blood pressure and IOP were measured to calculate ocular perfusion pressure (PP).
Results: cGMP and NO2 − plasma levels were significantly decreased in glaucoma patients compared with controls (p = 0.001 v p = 0.004). In the aqueous humour of subjects with POAG, cGMP and NO2 − concentrations were also lower than in normal eyes (p = 0.0001 v p = 0.001). There was a linear association between cGMP in plasma and aqueous humour in glaucomas and controls (r = 0.514, p = 0.029 and r = 0.558, p = 0.004) and this relation differed in the two groups (p = 0.003). Considering glaucoma patients with controls, a positive correlation was found between cGMP and PP (r = 0.379, p = 0.01) and between NO2 − and PP (r = 0.339, p = 0.040). The cGMP/PP correlation was of borderline statistical significance in controls (p = 0.050), whereas it did not attain statistical significance in POAG, as well as the association between NO2 − and PP when glaucomas and controls were considered separately.
Conclusions: The authors found alterations of NO markers in the plasma and aqueous humour of glaucoma patients. Primary or secondary impaired NO balance could alter ocular perfusion pressure.
Keywords: primary open angle glaucoma, nitric oxide, ocular perfusion pressure
Nitric oxide (NO) is synthesised in endothelial cells by type III isoform of nitric oxide synthase (NOS) and, under basal conditions of flow, it sustains a constant vasodilator tone in the vascular system. Changes in the environment, such as increased stress or hypoxia, can stimulate NO synthesis and the local regulation of vascular tone and blood flow. 1
Abundant NOS immunoreactivity has been found in the optic nerve head (ONH) vasculature. 2, 3 At this level an increase of NOS in the vascular endothelium has been hypothesised to be neuroprotective by promoting vasodilation and tissue blood flow. 3– 7
Nitric oxide synthase inhibition has been shown to strongly reduce choroidal and ONH blood flow in animals 8– 12 and humans. 13 Intravenous infusion of N G-nitro-L-arginine (L-NMMA), a NOS inhibitor, provoked a reduction of ONH blood flow in healthy subjects. 13 Another work reported that the NO donor 5-isosorbide mononitrate significantly increases ONH blood flow in normal subjects mainly causing an increment in blood volume, 14 and an elective sensitivity of the ONH circulation to nitrates has been postulated. 15
Tissue ischaemia has been involved in the pathogenesis of glaucomatous optic neuropathy and an altered circulation has been indicated in some glaucoma patients. 16– 21
Plasma and aqueous humour levels of cGMP, an indirect indicator of NO, were decreased in patients with normal tension glaucoma. The same glaucomatous group showed lower systolic and diastolic velocities of the ophthalmic artery when examined with colour Doppler imaging. 22 In POAG, abnormal IOP and vascular dysregulation might together determine optic nerve damage, both reducing ocular perfusion.
As NO can modulate IOP and ocular circulation, 23, 24 and contrasting data have been published recently on NO production 25, 26 in glaucoma patients, we wanted to elucidate whether NO formation is disturbed in hypertensive POAG and whether it influences ocular perfusion pressure. We therefore investigated the levels of NO2 −, the stable end product of NO metabolism, and of cGMP, the intracellular mediator of NO action, in aqueous humour and plasma of patients with POAG and controls.
MATERIALS AND METHODS
Study population
Thirty eight POAG patients were enrolled in the study together with 46 control subjects referred to our clinic for cataract surgery. Patients and controls were matched for age, sex, and blood pressure (table 1 ). The following exclusion criteria were used: any ocular disease except senile cataract or POAG; diabetes; infections; cardiovascular pathologies including hypertension and atherosclerotic lesions determining haemodynamically significant stenosis; assumption of non-steroidal anti-inflammatory drugs; steroids; ACE inhibitors, or NO donors. All subjects had to follow a nitrate free diet for three days before blood extraction.
Table 1.
Demographic characteristics of patients enrolled in the study
| Controls | POAG | p Value | |
| Number | 46 | 38 | |
| Mean (SD) age (years) | 71 (8.31) | 69.4 (8.43) | 0.394 |
| Sex | 25 women | 20 women | 0.950 |
| 21 men | 18 men | ||
| Mean (SD) blood pressure | |||
| Systolic | 139.6 (14.24) | 139.1 (11.71) | 0.863 |
| Diastolic | 79.5 (6.78) | 78.3 (11.71) | 0.559 |
| IOP (mm Hg) | 14.1 (2.8) | 16.6 (5.1) | 0.006 |
Glaucoma patients had IOP higher than 21 mm Hg without antiglaucomatous drug, open anterior chamber angle, glaucomatous disc damage (cup:disc ratio ⩾0.6), and glaucomatous field loss on the Humphrey perimeter, 30–2 full threshold programme (Humphrey Instruments, San Leandro, CA, USA). Visual field defects were included in the stage I–III, according to Aulhorn’s classification modified by Greve. 27 All control subjects had a normal ocular examination, IOP within normal limits, and intact visual fields.
Written informed consent was obtained from each subject and the tenets of the Declaration of Helsinki were observed.
Measurement of cGMP and NO2 − in the plasma and in the aqueous humour
Blood samples of 3 ml were collected from a forearm vein of every patient and immediately subjected to plasma separation and stored at −20°C.
Glaucoma patients and controls admitted to our clinic for cataract surgery, who met the aforementioned inclusion criteria, were also recruited for cGMP and NO2 − determination in the aqueous humour. Preoperatively, the same antibiotic and the same standard mydriatic protocol was used (three or four drops of an association of tropicamide 1% and phenylephrine 2.5%) for glaucomas and controls, so the two subgroups differed only for the antiglaucomatous treatment (timolol 0.50% twice a day). Cataract extraction was performed with a phacoemulsification technique. Before performing the clear corneal tunnel, 200–400 μl of aqueous humour was quickly withdrawn by means of a paracentesis, and replaced by a viscoelastic substance. The paracentesis were always performed by the same surgeon with a tuberculine needle on a 1 ml syringe. Ten μl of a solution of 10−5 M of isobutyl methylxantine (IBMX), a phosphodiesterase inhibitor, was added to each sample. All samples were stored at −20°C.
NO levels were evaluated in plasma and aqueous humour by measuring the concentration of NO2 − and of cGMP.
NO2 − was measured spectrophotometrically by the Griess reaction; 28 values were obtained by comparison with standard concentrations of sodium nitrite and expressed as micromoles of NO2 − per milligram of protein.
cGMP levels were measured in plasma and aqueous humour after the extraction with 10% trichloroacetic acid with 0.5 M tri-n-octylamine dissolved in 1,1,2-trichlorotrifluorethane. 28 cGMP was determined with the use of a radioimmunoassay kit (Amersham, Buckinghamshire, UK) after acetylation of the samples with acetic anhydride. Values are expressed as picomoles and as femtomoles of cGMP per milligram of protein for plasma and aqueous humour, respectively.
Protein concentrations were determined according to Lowry. 29 All experiments were performed in duplicates by a blind operator.
Ocular perfusion pressure
Immediately before collecting the blood sample, the blood pressure was measured by sphyngmomanometry. Although patients with a previous medical diagnosis of arterial hypertension were not enrolled at all in the study, we also excluded for ocular perfusion pressure evaluation subjects who had arterial pressure higher than 145/90 mm Hg before plasma withdrawal.
Intraocular pressure was assessed by Goldmann applanation tonometry (Haag Streit, Bern, Switzerland). Mean brachial artery blood pressure (BPm) was calculated according to the formula:
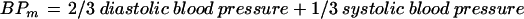 |
and ocular perfusion pressure (PP) was derived using the following relation:
 |
In control subjects and in patients with bilateral glaucoma the eye considered for PP calculation was chosen by random.
Statistical analysis
Results are shown as mean (standard deviation, SD). Statistical comparisons were conducted using the Student’s t test for unpaired observations. Linear regression and correlation were used to analyse the association between cGMP plasma and aqueous humour levels and between cGMP, NO2 − plasma concentration and PP. Comparisons between regression lines of glaucomas and controls were performed with the overall test for coincidence. A p value of ⩽0.05 was considered significant.
RESULTS
cGMP and NO2 − plasma levels
Forty six controls and 38 POAG subjects were eligible for cGMP assessment and among them, respectively, 19 and 23 also underwent NO2 − determination. cGMP plasma concentration was significantly lower in glaucoma patients than in normals (p = 0.001, fig 1 ).
Figure 1.
cGMP plasma levels in control (2059.68 (697.86) pmoles/mg protein) and glaucoma subjects (1568.62 (625.55) pmoles/mg protein). Values are mean (SD); p = 0.001 by the Student’s t test for unpaired data.
Also NO2 − plasma levels were decreased in POAG patients compared with controls (p = 0.004, fig 2 ).
Figure 2.
Nitrite (NO2 −) plasma concentrations in glaucomas (166.68 (38.45) μmol/mg protein) and controls (228.39 (76.22) μmoles/mg protein). Values are mean (SD); p = 0.004 by the Student’s t test for unpaired data.
cGMP and NO2 − levels in the aqueous humour
Aqueous humour samples were collected from 18 POAG patients and 25 subjects with cataract only. The glaucoma patients had a good tonometric control with therapy. Preoperative IOP statistically differed in the two groups (16 (4.33) v 13 (3.15) mm Hg; p = 0.012).
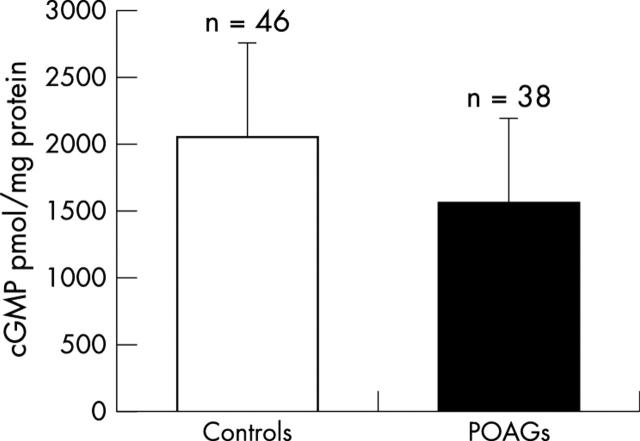
In glaucomatous eyes, cGMP mean concentration was significantly lower than in control eyes (p = 0.0001, fig 3 ).
Figure 3.
Amount of cGMP in the aqueous humour of patients with POAG and cataract (1559.32 (619.12) fmol/mg protein) v patients with cataract only (controls) (2931.07 (1387.01) fmol/mg protein). Student’s t test for unpaired data: p = 0.0001.
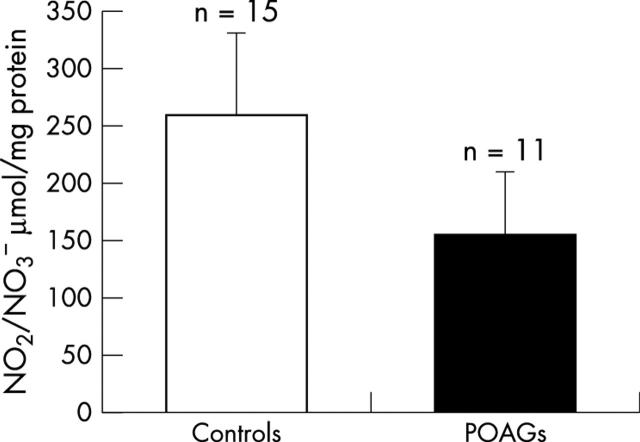
NO2 − amount was determined in the aqueous humour every time we could safely withdraw a sufficient volume from the anterior chamber of the individual eye. NO2 − was also notably reduced in eyes in POAG when compared with normotensive eyes (p = 0.001, fig 4 ).
Figure 4.
NO2 − levels in the aqueous humour of controls (259.20 (72.62) μmol/mg protein) and patients with glaucoma (156.82 (54.79) μmol/mg protein). Student’s t test for unpaired data: p = 0.001.
Association between cGMP in plasma and aqueous humour
A linear correlation between cGMP levels in plasma and aqueous humour (n = 43, r = 0.565, p<0.001) was found.
The association between plasmatic and aqueous humour cGMP concentrations was also statistically significant when calculated in the two groups separately (POAG group: n = 18, r = 0.514, p = 0.029; control group: n = 25, r = 0.558, p = 0.004) (fig 5 ).
Figure 5.
Linear regression between cGMP plasma levels and cGMP concentrations in the aqueous humour.
The relation between plasma and aqueous cGMP significantly differed in glaucoma and control patients (overall test of coincidence, p = 0.003; comparison of slopes, p = 0.001).
Ocular perfusion pressure
Perfusion pressure was calculated in 23 of the 38 glaucoma patients and in 22 of the 46 controls. Mean ocular PP was similar in POAGs (51.21 (5.66) mm Hg) and in normals (53.26 (6.40) mm Hg; p = 0.262).
Considering glaucomas and controls together, there was a linear, positive correlation between cGMP plasma levels and PP (n = 45, r = 0.379, p = 0.01), as well as between plasmatic NO2 − s and PP (n = 37, r = 0.339, p = 0.04). When the association of plasma cGMP and PP was explored in glaucoma patients and in controls, a positive correlation between the two variables was found in both groups (r = 0.249 and r = 0.423, respectively), but this relation did not attain statistical significance in POAG, whereas it was of borderline statistical significance in the control group (p = 0.050, fig 6 ). No statistically significant correlation was found between plasma NO2 − levels and PP when glaucomas and controls were evaluated separately. No significant difference was found in the two groups of patients with respect to the association plasma cGMP with PP or plasma NO2 − and PP.
Figure 6.
Linear regession between cGMP plasma levels and ocular perfusion pressure (PP).
DISCUSSION
In the present study, decreased concentrations of NO2 − and cGMP were found in the plasma and aqueous humour of glaucoma patients. Our data on PP show that plasma NO correlates with PP, in that higher levels of cGMP and NO2 − are associated with higher PP values.
The lower plasma levels of NO indicators in patients with POAG could reflect an impaired balance of endothelium derived mediators in glaucoma, as suggested by other evidence. 21, 30
Decreased plasma cGMP seems to correlate with lower cGMP in the aqueous humour. As NO has a very short half life (2–30 s1), and production of NO in the anterior chamber has been documented, 24, 31, 32 NO in aqueous humour should mainly derive from local formation. For this reason, the association found between plasma and aqueous humour cGMP is interesting, and suggests that a basic disorder in NO balance may act both systemically and locally. Our findings in the aqueous humour agree with those of other authors, 25 and with the report of decreased NOS reactivity in the aqueous outflow pathway of postmortem glaucomatous eyes. 33 Such alterations, together with pharmacological studies showing that NO donors reduce IOP elevating NO2 − in the anterior chamber, 24 suggest that NO might be involved in the pathogenesis or regulation of IOP in POAG.
Higher IOP could alter NO concentration in the aqueous humour, but not in peripheral blood. Ocular antihypertensive medications such as β blockers could have an influence on NO levels in the aqueous humour and plasma. Although the β blocker propanolol inhibited the nitrite production evoked by a β adrenergic receptor agonist, it had no inhibitory effect either on NO formation induced by mediators other than β adrenergic agonists, or on basal production of NO. 34 Moreover, NOS reactivity of glaucomatous eyes which had never received any antiglaucomatous medications showed severe alterations, similar to those found in the treated eyes. 33 The effects of systemic β blockers on plasma NO levels are still unclear, even if some β blockers, such as nebivolol, are known to induce arterial and venous dilatation by greatly increasing cGMP. 35, 36
Impaired NO formation can have a dual negative effect in glaucoma patients by acting on IOP and ocular blood supply, as shown by the relation between plasma cGMP or NO2 − and PP. The correlation between the two parameters found in controls indicates that decreased NO levels are associated with lower PP. Nitric oxide could influence PP modulating both IOP and blood pressure. Lower PP is strongly associated with an increased prevalence of POAG. 37 An impaired ONH autoregulation makes the glaucomatous optic nerve more sensitive to decreased PP. 19 Recently, NO has been involved in choroidal autoregulation. 38 In our study, the relation between plasma cGMP or NO2 − and PP did not attain statistical significance in glaucomas. This could be because of the small data set, or to glaucoma therapy, that makes IOP less affected by physiological stimuli. Otherwise, an altered NO formation could modify NO influence on PP. This hypothesis would fit well with the report that glaucoma patients receiving nitrate therapy for systemic conditions have less progression of glaucomatous optic neuropathy and visual field loss than patients who do not take these compounds. 39
Acknowledgments
Research winner, the Italian selection of the Merck Sharp & Dohme International Award 2002.
Abbreviations
cGMP, cyclic guanosine monophosphate
NO, nitric oxide
NOS, nitric oxide synthase
POAG, primary open angle glaucoma
PP, perfusion pressure
REFERENCES
- 1. Lincoln J, Hoyle CHV, Burnstock G. Nitric Oxide in health and disease. Cambridge University Press, 1997.
- 2. Qi X, Guy J. Localization of NADPH diaphorase/nitric oxide synthase in the optic nerve of the normal guinea pig: a light and electron microscopic study. J Comp Neurol 1996;370:396–404. [DOI] [PubMed] [Google Scholar]
- 3. Neufeld AH, Hernandez MR, Gonzalez M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch Ophthalmol 1997;115:497–503. [DOI] [PubMed] [Google Scholar]
- 4. Haefliger IO, Flammer J, Lüscher TF. Nitric oxide and endothelin 1 are important regulators of human ophthalmic artery. Invest Ophthalmol Vis Sci 1992;33:2340–3. [PubMed] [Google Scholar]
- 5. Haefliger IO, Flammer J, Lüscher TF. Heterogeneity of endothelium-dependent regulation in ophthalmic and ciliary arteries. Invest Ophthalmol Vis Sci 1993;34:1722–30. [PubMed] [Google Scholar]
- 6. Meyer P, Flammer J, Lüscher TF. Endothelium-dependent regulation of the ophthalmic microcirculation in the perfused porcine eye: role of nitric oxide and endothelins. Invest Ophthalmol Vis Sci 1993;34:3614–21. [PubMed] [Google Scholar]
- 7. Koss MC. Functional role of nitric oxide in regulation of ocular blood flow. Eur J Pharmacol 1999;374:161–74. [DOI] [PubMed] [Google Scholar]
- 8. Deussen A, Sonntag M, Vogel R. L-Arginine-derived nitric oxide: a major determinant of uveal blood flow. Invest Ophthalmol Vis Sci 1993;34:129–34. [DOI] [PubMed] [Google Scholar]
- 9. Mann RM, Riva CE, Stone RA, et al. Nitirc oxide and choroidal blood flow regulation. Invest Ophthalmic Vis Sci 1995;36:925–30. [PubMed] [Google Scholar]
- 10. O’Brien C, Kelly PA, Ritchie IM. Effect of chronic inhibition of NO synthase on ocualr blood flow and glucose metabolism in the rat. Br J Ophthalmol 1997;81:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buerk DG, Riva CE, Cranstoun SD. Nitric oxide has a vasodilatory role in the cat optic nerve head during flicker stimuli. Microvasc Res 1996;52:13–26. [DOI] [PubMed] [Google Scholar]
- 12. Sugiyama T, Oku H, Ikari S, et al. Effect of nitric oxide synthase inhibitor on optic nerve head circulation in conscious rabbits. Invest Ophthalmic Vis Sci 2000;41:1149–52. [PubMed] [Google Scholar]
- 13. Luksch A, Polak K, Beier C, et al. Effects of systemic NO synthase inhibition on choroidal and optic nerve head blood flow in healthy subjects. Invest Ophthalmic Vis Sci 2000;41:3080–84. [PubMed] [Google Scholar]
- 14. Grunwald JE, Iannacone A, DuPont J. Effect of isosorbide mononitrate on the human optic nerve and choroidal circulations. Br J Ophthalmol 1999;83:162–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iannacone A, DuPont J, Grunwald JE. Human retinal hemodynamics following administration of 5-isosorbide mononitrate. Curr Eye Res 2000;20:205–10. [PubMed] [Google Scholar]
- 16. Galassi F, Sodi A, Rossi MG, et al. Results of color Doppler imaging in various types of glaucoma. In: Pillunat LE, Harris A, Anderson DR, Greve EL, eds. Current Concepts on Ocular Blood Flow in Glaucoma. The Hague, The Netherlands: Kugler Publication, 1999:119–27.
- 17. Flammer J, Haeflinger IO, Orgül S, et al. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma 1999;8:212–19. [PubMed] [Google Scholar]
- 18. Flammer J, Orgül S. Optic nerve blood flow abnormalities in glaucoma. Progr Retinal Eye Res 1998;17:267–89. [DOI] [PubMed] [Google Scholar]
- 19. Drance SM. The Vascular Factors in Glaucoma. In: Bucci MG, ed. Glaucoma: Decision Making in Therapy. Berlin/Heidelberg/New York: Springer-Verlag, 1996:31–5.
- 20. Kaiser HJ, Flammer J, Wenk M, et al. Endothelin-1 plasma levels in normal-tension glaucoma: abnormal response to postural changes. Graefes Arch Clin Exp Ophthalmol 1995;233:484–8. [DOI] [PubMed] [Google Scholar]
- 21. Henry E, Newby DE, Webb DJ, et al. Peripheral endothelial dysfunction in normal pressure glaucoma. Invest Ophthalmol Vis Sci 1999;40:1710–14. [PubMed] [Google Scholar]
- 22. Galassi F, Sodi A, Ucci F, et al. Ocular haemodynamics and nitric oxide in normal pressare glaucoma. Acta Ophthalmol Scand 2000;78 (S232):37–38. [DOI] [PubMed] [Google Scholar]
- 23. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci 1994;35:2515–20. [PubMed] [Google Scholar]
- 24. Chuman H, Chuman T, Nobuhisa N, et al. The effect of L-arginine on intraocular pressure in the human eye. Curr Eye Res 2000;20:511–16. [PubMed] [Google Scholar]
- 25. Doganay S, Evereklioglu C, Turkoz Y, et al. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol 2002;12:44–8. [DOI] [PubMed] [Google Scholar]
- 26. Kotikoski H, Moilanen E, Vapaatalo H, et al. Biochemical markers of the L-arginine-nitric oxide pathway in the aqueous humour in glaucoma patients. Acta Ophthalmol Scand 2002;80:191–5. [DOI] [PubMed] [Google Scholar]
- 27. Greve EL, Langerhost CT, van den Berg TTJP. Perimetry and other visual function tests in glaucoma. In: Cairns JE, ed. Glaucoma 1. London: Grune and Stratton, 1986:37–77.
- 28. Bani D, Masini E, Bello MG, et al. Relaxin activates the L arginine-nitric oxide pathway in human breast cancer cells. Cancer Res 1995;55:5272–5. [PubMed] [Google Scholar]
- 29. Lowry OH, Rosenbrough NJ, Farr AL, et al. Protein measurement with folin-phenol reagent. J Biol Chem 1951;193:265–75. [PubMed] [Google Scholar]
- 30. Haefeli W, Linder L, Gass A, et al. Peripheral microcirculatory responses to vasoconstrictors in glaucoma patients. In: Haefliger IO, Flammer J, eds. Nitric Oxide and endothelin in the pathogenesis of glaucoma. Philadelphia: Lippincott-Raven, 1998:102–11.
- 31. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci 1995;36:1765–73. [PubMed] [Google Scholar]
- 32. Tamm ER, Flügel-Koch C, Mayer B, et al. Nerve cells in the human ciliary muscle: ultrastructural and immunocytochemical characterization. Invest Ophthalmol Vis Sci 1995;36:414–26. [PubMed] [Google Scholar]
- 33. Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci 1995;36:1774–84. [PubMed] [Google Scholar]
- 34. Liu R, Flammer J, Haefliger IO. Isoproterenol, Forskolin, and cAMP-induced nitric oxide production in pig ciliary processes. Invest Ophthalmol Vis Sci 1999;40:1833–7. [PubMed] [Google Scholar]
- 35. Gao Y, Nagao T, Bond RA, et al. Nebivolol induces endothelium-dependent relaxations of canine arteries. J Cardiovasc Pharmacol 1991;17:964–9. [DOI] [PubMed] [Google Scholar]
- 36. Bowman AJ, Chen CPLH, Ford GA. Nitric oxide mediated venodilator effect of nebivolol. Br J Clin Pharmacol 1994;38:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Opthalmol 1995;113:216–21. [DOI] [PubMed] [Google Scholar]
- 38. Luksch A, Polska E, Imhof A, et al. Role of NO in choroidal blood flow regulation during isometric exercise in healthy humans. Invest Ophthalmol Vis Sci 2003;44:734–9. [DOI] [PubMed] [Google Scholar]
- 39. Zurakowski D, Vortwerk CK, Gorla M, et al. Nitrate therapy may retard glaucomatous optic neuropathy, perhaps through modulation of glutamate receptors. Vis Res 1998;38:1489–94. [DOI] [PubMed] [Google Scholar]