Metastatic choroidal melanoma typically presents in the liver. Therefore, liver enzyme assays are the most common haematological evaluation performed after treatment.1
In 1985, The Collaborative Ocular Melanoma Study required periodic medical evaluations including a physical examination, liver functions studies, a complete blood count, and a chest x ray. If liver enzymes exceeded 1.5 times normal, computed tomography (CT) of the abdomen was required. If low attenuation hepatic nodules suggested metastatic disease, fine needle aspiration biopsy of the liver tumours provided cytopathological confirmation.1
Positron emission tomography (PET) is a molecular imaging technique that uses radiolabelled molecules to image metabolic activity in vivo.2,3 When whole body PET was combined with computed radiographic tomography (CT), PET/CT put anatomy and function on the same page making practical a new era of physiological imaging.2–6
This study examines the ability of positron emission tomography combined with computed tomography (PET/CT) to allow for detection of previously occult metastatic melanoma.
Case report
A 77 year old woman presented with a 15.4×15 mm width and 13.2 mm high collar button shaped choroidal melanoma with a large secondary retinal detachment in her right eye. Her preoperative medical evaluation (including CT imaging of the abdomen) proved negative. She was treated by enucleation.
Two years later a follow up medical evaluation revealed elevated liver function studies (table 1) and a chest x ray showed a pleural effusion. CT of the abdomen with contrast revealed multiple low attenuation hepatic foci consistent with metastatic melanoma.
Table 1.
Patient characteristics
| Physical | Examination | Blood examination | X ray | CT scan abdomen with contrast | Whole body PET/CT | |
| CT | PET | |||||
| General | Icterus | |||||
| Subcutaneous tissue | 2 nodules in the anterior abdominal wall | No lesions noted | 1.4 cm subcutaneous nodule in the anterior abdominal wall | Hypermetabolic focus in the subcutaneous tissue of the anterior abdominal wall | ||
| Nodes | None | No lesions noted | Enlarged para aortic lymph nodes on the left side | Hypermetabolic focus in the upper abdomen | ||
| Lungs | No abnormalities noted | Pleural effusion of right lung base. No nodules | Pleural effusion right lung base and calcified hilar nodes | Two small 3 mm nodules in the right upper lobe. Right pleural effusion | No foci noted | |
| Liver | Enlarged | High bilirubin, AST, ALT, alkaline phosphatase, GGT | Enlarged. 2 areas of low attenuation in the anterior aspect of the right lobe | Low attenuation lesion >20 cm in greatest diameter in the right lobe with calcification seen posteriorly. Numerous low attenuation lesions throughout both lobes of the liver | Enlarged. Large hypermetabolic focus in the right lobe with mass effect. Numerous hypermetabolic foci throughout remainder of liver. | |
| Kidney | 8 mm cyst midpole and 2 cm cyst upper pole of left kidney | Large right renal cyst. Additional smaller cysts | Photopenic defect due to large right renal cyst | |||
| Bone | No lesions noted | No lesions noted | Hypermetabolic foci in the right skull base, left scapula, left humerus, sternum, multiple bilateral ribs, thoracic and lumbosacral spine, pelvis, and bilateral femurs | |||
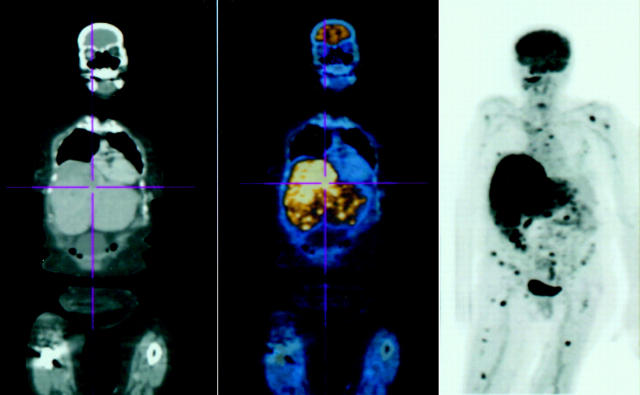
A PET/CT was requested. Fifty minutes after intravenous administration of 15.2 mCi of fluordeoxyglucose, whole body PET/CT imaging revealed enlarged para-aortic lymph nodes and a subcutaneous nodule in the abdominal wall (fig 1). The CT portion of the PET/CT also revealed two 3 mm nodules in the upper lobe of the right lung (too small to be visualised by PET imaging). PET imaging was able to reveal multiple bony metastasis that were not seen on the CT portion (fig 1). Both CT and PET showed a large liver metastasis, but CT was better at defining tumour size. Since it is a physiological assay, PET also demonstrated the metabolic activity of the metastatic tumours (fig 1).
Figure 1.
On the left, the CT demonstrates the anatomy; on the right the PET shows areas of hypermetabolism (glucose uptake); in the middle the two images are fused. PET/CT revealed enlarged para-aortic lymph nodes and a subcutaneous nodule in the anterior abdominal wall. The PET imaging portion of the PET/CT was able to reveal multiple bony metastases that were not seen on the CT portion of the examination. Both CT and PET showed a large liver metastasis.
Comment
In this case, whole body PET/CT was found to be capable of uncovering metastases not seen with abdominal CT alone. This led us away from considering regional perfusion of the liver, hepatic resection, and towards systemic treatment.7,8 Therefore, when PET/CT identifies extrahepatic involvement, it can have a significant impact on the management of patients with metastatic choroidal melanoma.
PET/CT could also be used for initial staging. Early detection of occult metastases offers the potential to avoid ineffective and expensive enucleations, radioactive plaques, proton irradiation, eye wall resections, or laser treatment.9,10 Local therapies would be abandoned in favour of systemic treatments.
Another issue related to PET/CT is cost. Up to five times more than CT of the abdomen, PET/CT is only covered (Medicare) for melanoma staging/restaging when the stage of the cancer remains in doubt after completion of conventional imaging (or if the clinical management would differ depending on the PET findings). Since PET/CT revealed extrahepatic foci in this case, it changed our clinical approach. There is little doubt about the improved ability of PET/CT to detect lesions; the real issue is cost and if the results will change outcomes.
This study goes one step further than CT, MRI, or PET alone. By combining whole body PET and CT, this examination joins anatomy and function in one examination (fig 1). The relative efficacy of PET/CT to locate metastases should be evaluated within the framework of a prospective study.
This work was supported by The EyeCare Foundation and Research to Prevent Blindness, New York, USA.
References
- 1.Diener-West M , Hawkins BS, Markowitz JA, et al. A review of mortality from choroidal melanoma. II A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch Ophthalmol 1992;110:245–50. [DOI] [PubMed] [Google Scholar]
- 2.Townsend DW, Cherry SR. Combined anatomy and function: the path to true image fusion. Eur Radiol 2001;11:1968–74. [DOI] [PubMed] [Google Scholar]
- 3.Mijnhout GS, Hoekstra OS, van Tulder MW, et al. Systematic review of the diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography in melanoma patients. Cancer 2001;91:1530–42. [PubMed] [Google Scholar]
- 4.Stas M , Stroobants S, Dupont P, et al. 18-FDG PET scan in the staging of recurrent melanoma: additional value and therapeutic impact. Melanoma Res 2002;12:479–90. [DOI] [PubMed] [Google Scholar]
- 5.Swetter SM, Carroll LA, Johnson DL, et al. Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol 2002;9:646–53. [DOI] [PubMed] [Google Scholar]
- 6.Finger PT, Czechonska G, D’Arienzo P, et al. Positron emission tomography of choroidal melanoma. Probl Med Nukl 1996;10:235–9. [Google Scholar]
- 7.Mavligit GM, Charnsangavej C, Carrasco CH, et al. Regression of ocular melanoma metastatic to the liver after hepatic arterial chemoembolization with cisplatin and polyvinyl sponge. JAMA 1988;260:974–6. [PubMed] [Google Scholar]
- 8.Albert DM, Niffenegger AS, Willson JK. Treatment of metastatic uveal melanoma: review and recommendations. Surv Ophthalmol 1992;36:429–38. [DOI] [PubMed] [Google Scholar]
- 9.Moshfeghi DM, Moshfeghi AA, Finger PT. Enucleation. Surv Ophthalmol 2000;44:277–301. [DOI] [PubMed] [Google Scholar]
- 10.Finger PT. Radiation therapy for choroidal melanoma. Surv Ophthalmol 1997;42:215–32. [DOI] [PubMed] [Google Scholar]