Intravitreal and sub-Tenon’s corticosteroids are being evaluated for the treatment of choroidal neovascularisation (CNV).1–3 We reported on the efficacy of triamcinolone acetonide administered as a trans-Tenon’s retrobulbar infusion (triamcinolone infusion) in reducing inflammation in uveitic eyes.4 Here we evaluated the same treatment in eyes with small subfoveal CNV.
Case reports
Triamcinolone infusion was performed in 22 eyes of 22 patients with subfoveal CNV of greatest diameter less than or equal to 1 disc diameter (DD). The diagnoses were age related macular degeneration (AMD) in 14 eyes, idiopathic CNV in four eyes, polypoidal choroidal vasculopathy (PCV) in three eyes, and punctate inner choroidopathy (PIC) in one eye. One AMD eye had undergone ablative argon laser photocoagulation for CNV previously, but no other eyes had received previous treatment. The median post-triamcinolone infusion follow up was 7.5 months (range 4–27 months). Pretreatment fluorescein angiography (FA) revealed predominantly classic CNV in 12 eyes and predominantly occult CNV in 10 eyes. Records were reviewed retrospectively and did not require institutional review board approval. Informed consent was obtained before each procedure.
The patient’s eye was prepared with povidone-iodine and sterile drapes applied. After topical anaesthesia, conjunctiva and Tenon’s capsule were incised in the inferotemporal quadrant. A 23 gauge curved blunt cannula approximately 2.1 cm in length (#HS-2764, Handaya Co, Ltd, Tokyo, Japan) was inserted to the hub into sub-Tenon’s space and 20 mg (0.5 ml) triamcinolone acetonide (Bristol Pharmaceutical, KK, Tokyo, Japan) was infused. The wound was left unsutured and 0.5% levofloxacin was instilled topically three times a day for 1 week.
Onset of CNV fibrosis was observed in 14 eyes (64%) by 3 months post-triamcinolone infusion (fig 1). Rates of fibrosis were 50% (7/14 eyes) for AMD, 100% (4/4 eyes) for idiopathic CNV, 67% (2/3 eyes) for PCV, and 100% (1/1 eye) for PIC. Fibrosis did not correlate with CNV size or lesion composition. FA performed at 3 months showed decreased lesion leakage in 12 eyes (55%), no change in five eyes (23%), and increased leakage in five eyes (23%). Best corrected visual acuity (VA) at 3 months for all eyes improved by ⩾0.2 logarithm of the minimum angle of resolution (logMAR) in four eyes (18%), remained unchanged in 13 eyes (59%), and worsened by ⩾0.2 logMAR in five eyes (23%). The median decimal VA for all eyes was 0.30 before treatment (range 0.08–1.0) and 0.24 at 3 months after treatment (range 0.05–1.2). Of the 14 eyes with AMD, the VA at 3 months improved by ⩾0.2 logMAR in one eye (7%), remained unchanged in 10 eyes (71%), and worsened by⩾0.2 logMAR in three eyes (21%). In these AMD eyes, the median decimal VA was 0.30 before treatment (range 0.08–1.0) and 0.20 at 3 months after treatment (range 0.05–0.7). Complications such as intraocular pressure elevation, infection, or cataract progression were not noted in any eyes.
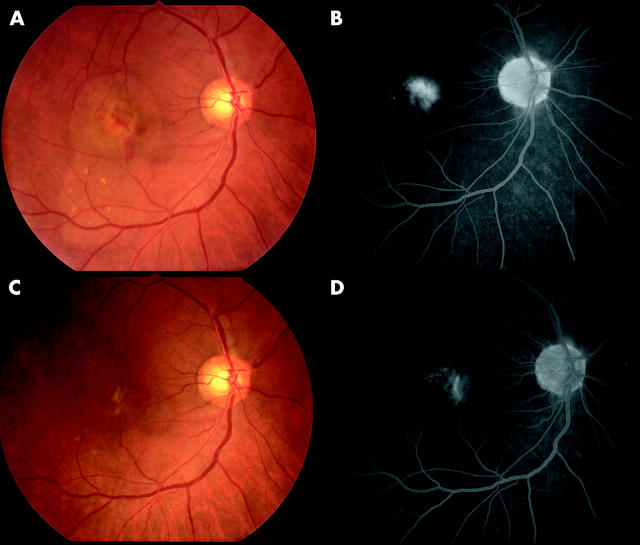
Figure 1.
Colour fundus photographs and fluorescein angiography late images of an eye in a 65 year old patient with age related macular degeneration and small subfoveal choroidal neovascularisation before (A, B; best corrected visual acuity 0.3) and 3 months after triamcinolone infusion (C, D; best corrected visual acuity 0.7).
Comment
This interventional case series shows that trans-Tenon’s retrobulbar infusion of triamcinolone acetonide resulted in lesion fibrosis in the majority of eyes with small CNV, efficacy being best for idiopathic CNV or CNV related to PIC. The mechanism of action of triamcinolone acetonide in inhibiting CNV growth probably involves several pathways. The effect of corticosteroids in inhibiting inflammatory cells that participate in the neovascular response probably has a prominent role.5,6 Triamcinolone acetonide has specifically been shown to inhibit basic fibroblast growth factor induced migration and tube formation of choroidal microvascular endothelial cells.7 Furthermore, triamcinolone acetonide inhibits choroidal neovascularisation induced by laser trauma in a rat model.8 Finally, triamcinolone acetonide may decrease vascular permeability, thereby decreasing influx of serum proteins that may contribute to an angiogenic microenvironment.9 Longer follow up and greater numbers of cases in a randomised clinical trial are needed to confirm these results.
References
- 1.Challa JK, Gillies MC, Penfold PL, et al. Exudative macular degeneration and intravitreal triamcinolone: 18 month follow-up. Aust NZ J Ophthalmol 1998;26:277–81. [DOI] [PubMed] [Google Scholar]
- 2.Danis RP, Ciulla TA, Pratt LM, et al. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina 2000;20:244–50. [PubMed] [Google Scholar]
- 3.Gillies MC, Simpson JM, Luo W, et al. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: one year results. Arch Ophthalmol 2003;121:667–73. [DOI] [PubMed] [Google Scholar]
- 4.Okada AA, Wakabayashi T, Morimura Y, et al. Trans-Tenon’s retrobulbar triamcinolone infusion for the treatment of uveitis. Br J Ophthalmol 2003;87:968–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proia A , Hirakata A, McInnes J, et al. The effect of angiostatic steroids and B-cyclodextrin tetradecasulfate on corneal neovascularization in the rat. Exp Eye Res 1993;57:693–8. [DOI] [PubMed] [Google Scholar]
- 6.Oh H , Takagi H, Takagi C, et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1999;40:1891–8. [PubMed] [Google Scholar]
- 7.Wang Y , Friedrichs U, Eichler W, et al. Inhibitory effects of triamcinolone acetonide on bFGF-induced migration and tube formation in choroidal microvascular endothelial cells. Graefes Arch Clin Exp Ophthalmol 2002;240:42–8. [DOI] [PubMed] [Google Scholar]
- 8.Ciulla TA, Criswell MH, Danis RP, et al. Intravitreal traimcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch Ophthalmol 2001;119:399–404. [DOI] [PubMed] [Google Scholar]
- 9.Carnuccio R , Di Rosa M, Guerrasio B, et al. Vasocortin: a novel gluococorticoid-induced anti-inflammatory protein. Br J Pharmacol 1987;90:443–5. [DOI] [PMC free article] [PubMed] [Google Scholar]