Abstract
TraR, a member of the LuxR family of quorum-sensing transcription factors, is responsible for the population density-dependent regulation of Ti plasmid conjugal transfer. The protein requires as coinducer an acyl-homoserine lactone signal molecule called AAI (Agrobacterium autoinducer) that is produced by the bacteria themselves. TraR only activates its target genes, making it difficult to determine whether interaction with AAI is required for binding DNA or for initiating transcription. To assess this, we converted TraR into a repressor by placing a copy of the tra box, an 18-bp inverted repeat believed to be the recognition site for this protein, over the −10 region of a promoter driving expression of lacZ. Repression of this promoter by TraR depended on AAI or, at higher concentrations, VAI, the closely related signal of Vibrio fischeri. C-terminal deletions as short as 2 aa and N-terminal deletions as short as 4 aa in TraR abolished both repressor and activator functions. The C-terminal mutants were strongly dominant over TraR, suggesting that they can form heteromultimers with the wild-type activator. Mutants of TraR with substitutions at Asp-10 and Gly-123 failed to activate a positively controlled reporter but continued to repress the chimeric promoter in an AAI-dependent manner. We conclude that TraR recognizes the tra box as its binding site, that binding of TraR to this site depends on AAI, and that the N-terminal half of the protein contains one or more domains that are required for activation but not for multimerization, for interaction with the acyl-homoserine lactone, or for DNA binding.
Keywords: autoinduction, gene regulation, acyl-homoserine lactones
Quorum-sensing, a regulatory strategy used by many bacteria, coordinates expression of target genes with the size of the bacterial population. Among the well characterized examples, Agrobacterium tumefaciens controls conjugal transfer of its Ti plasmids by quorum-sensing (1, 2). In this system, the transcriptional activator TraR requires Agrobacterium autoinducer (AAI) for expression of the Ti plasmid tra regulon. AAI [N-(3-oxo-octanoyl)-l-homoserine lactone (3)], is the density-sensing signal; it is produced by the bacteria and accumulates in the habitat during growth of the donor population.
TraR is a member of the LuxR family of transcriptional activators. This latter protein, required for density-dependent expression of the lux operon of Vibrio fischeri (4), requires a related acyl-homoserine lactone called VAI [N-(3-oxo-hexanoyl)-l-homoserine lactone] (5). According to the current model, signal binding alters the conformation of the LuxR-like activators, allowing them to interact with their promoters, with RNA polymerase, or with both (6). However, little is known about how these activators associate with their promoters or the role played by the signal in this interaction.
Domains required for autorepression (6), VAI binding (7, 8), multimerization (9), DNA binding (6–8), and transcriptional activation (6, 10) have been located on LuxR. Similar assignments have not been made for other members of the family, although all share a helix–turn–helix (HTH) motif at the C terminus of the protein. LuxRΔN, an N-terminal deletion derivative that retains this region, induces lux expression, suggesting that the DNA-binding and activation domains of LuxR are located in the 88-residue C-terminal portion of the protein (6). Moreover, activation by LuxRΔN is independent of VAI, supporting the model in which, in the absence of the signal, the N-terminal domain prevents the protein from activating transcription (6).
TraR, being one of the smallest of the LuxR-like activators, is an excellent candidate for structure–function analyses. Promoters regulated by TraR contain one or more copies of an 18-bp inverted repeat called the tra box (11–13). Recently, this protein has been purified to homogeneity and shown to bind to the tra box, where it can initiate transcription in concert with RNA polymerase (14). However, functional TraR was isolated only from cells grown in the presence of AAI, so the influence of this signal on the activator remains to be determined.
Because most members of the LuxR family are activators, genetic analyses cannot discriminate between DNA-binding and transcriptional initiation. Repression of gene expression, on the other hand, provides a measure of DNA binding independent of activation. In this regard, LuxR weakly represses expression of its own gene. Moreover, under normal conditions, this autorepression depends on VAI (15, 16) suggesting that interaction with the signal is required for DNA binding. Unfortunately, TraR does not repress any known genes, making it difficult to discriminate between functions involved in transcriptional activation and DNA binding. In this report, we describe the construction of a lacZ reporter gene driven by an artificial promoter repressible by TraR. Using this promoter–reporter construct, we have examined the DNA-binding properties of TraR and its mutants and the influence of AAI on this binding.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions.
A. tumefaciens NT1 was grown at 28°C in LB broth (17), MG/L (18), or ABM minimal medium (19). Escherichia coli DH5α was grown at 37°C in LB or in A medium (20). Antibiotics, 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-Gal), and isopropyl-β-d-thiogalactopyranoside (IPTG) were included in medium at concentrations described (21). Unless otherwise specified, synthetic AAI (22) was added to culture medium to a concentration of 25 nM. Plasmid DNA was introduced into A. tumefaciens and E. coli strains as described (11).
Plasmid and Promoter Constructions.
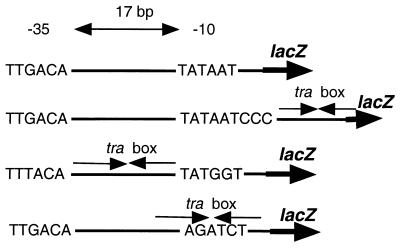
Promoters containing tra-box elements were constructed in pRG970b, a promoter-selection vector that contains a promoterless lacZ with its own ribosome-binding site (23). All promoters contain the consensus −35 element TTGACA except for pZLBet1, which contains the −35 and −10 elements of the E. coli lac promoter (Table 1). pZLR190, which does not contain a tra box, was constructed by cloning the Ant promoter of phage P22 from pPY190 (24) into pRG970b. This base promoter was modified by direct oligonucleotide cloning or by PCR. The sequences of all oligonucleotides used for direct constructions or as primers for PCR are available on request. pZLBox3, in which the tra box is located 3 bp downstream of the −10 site, was constructed by cloning into pZLR190 a SmaI–BamHI fragment produced by annealing two appropriate oligonucleotides. To position the tra box between the −35 and −10 sites, or to center the element over the −10 site, the appropriate complementary oligonucleotides were annealed and cloned into pRG970b digested by BamHI and SmaI to generate pZLBet1 and pPBL1, respectively. pZLQR, which expresses traR from Ptrc, was constructed by cloning the activator gene into pZLQ, a derivative of pBBRMCS-2 (25) containing lacIq.
Table 1.
Repression of promoters containing the tra box by TraR
Expressed as units per 108 colony-forming units. Assays were performed in E. coli. Indistinguishable patterns of expression were observed when the assays were performed in A. tumefaciens. All values are averages of three independent experiments with variations <15%.
For constructing the mutant repression reporters pPBL1-D and pPBL1-I, pPBL1 was digested with BglII, the site for which is located in the middle of the tra box, and treated with either mung bean nuclease to remove the 4-bp overhang, or with Klenow fragment of DNA polymerase to fill in the overhang. To construct pLMB4–15, in which the 4th and 15th nucleotides of the tra box are altered, complementary oligonucleotides with the desired substitutions were annealed and cloned into pRG970b.
For constructing the activation reporters, the 251-bp tra intergenic region between the ribosome-binding sites of traA and traC (11) was amplified by PCR and cloned into pTZBclI, a derivative of pTZ18U containing a BclI site, to generate pTZ251. The region was recloned into pRG970b as a BclI–SmaI fragment to produce pZLb251. This plasmid was used to construct pZLb251-I and pZLb251-D that contain 4-bp insertion and deletion mutations, respectively, at the BglII site of the tra box essentially as described above. Mutations at the 4th and 15th nucleotides of the tra box were introduced into pTZ251 by site-directed mutagenesis using the QuikChange Kit (Stratagene). The intergenic region containing the mutated tra box was cloned into pRG970b to give pZLbm4–15.
Deletion and Random Mutagenesis of traR.
Precise deletions were constructed by using PCR with Pfu DNA polymerase (Stratagene) and traR cloned in pZLQR as template. For N-terminal deletions, a C-terminal primer with the native translational stop codon was coupled with N-terminal primers containing an in-frame ATG as part of an NdeI site to amplify fragments coding for N-terminal shortened versions of TraR. For C-terminal deletions, an N-terminal primer with the native ATG in the form of an NdeI site was coupled with C-terminal primers containing an in-frame TGA stop codon placed to give versions of TraR terminating at successively earlier positions. In all cases, the PCR products were cloned as NdeI–EcoRI fragments into pZLQ, which contains an NdeI site within an initiation codon driven by the trc promoter. traR was mutagenized by treating purified pZLQR with hydroxylamine (10). All mutant genes were recloned into a fresh vector.
DNA Sequence Analysis.
The double-stranded nucleotide sequences of all promoter constructs, deletion derivatives, and hydroxylamine-induced mutants were verified by using automated sequencing by the University of Illinois Biotechnology Center.
Enzyme Activity Assays.
β-Galactosidase activity was quantified as described by Miller (20). For kinetic studies, overnight cultures were diluted 1:200 into fresh medium and incubated with shaking. When the culture density reached a Klett value (red filter) of about 15, AAI was added, incubation was continued, and samples were withdrawn for analysis at the indicated times. To determine dependence of TraR activity on the concentration of the acyl-homoserine lactone (acyl-HSL), early exponential-phase cultures prepared as described above were split into subcultures, and each was supplemented with the signal at the concentration noted. The cultures were incubated in parallel to an OD600 of 0.75–0.85, and the cells were harvested and assayed for β-galactosidase activity.
Protein Analysis.
Overnight cultures were diluted 1:100 in MG/L medium and incubated until the OD600 reached about 0.3. IPTG was added, and the cultures were grown to an OD600 of about 1.0. The cells were collected and lysed by boiling for 5 min in loading buffer (17). Equal amounts of protein from each lysate were electrophoresed in 15% SDS polyacrylamide gels, the gels were stained with Coomassie brilliant blue, and the proteins were transferred by electroelution to nitrocellulose membranes (Bio-Rad). TraR was detected by using murine polyclonal antiserum raised against affinity-purified 6-His-tagged activator, and the reacting bands were visualized by using goat anti-murine antibody (26).
RESULTS
TraR Can Function as a Transcriptional Repressor.
The mechanism by which TraR activates gene expression and the role of AAI in this process are not known. We reasoned that by converting TraR into a repressor, we could examine DNA binding independent of activation and determine the role of AAI in these two processes. We constructed versions of a plasmid-borne copy of a strong promoter fused to a lacZ reporter gene, each containing a copy of the tra box at a different location (Table 1). The unmodified promoter is very active in A. tumefaciens and E. coli. Placing the tra box between the −10 and −35 elements or positioning the box 3 bp downstream from the −10 element had only a modest effect on promoter activity (Table 1). However, centering the tra box over the −10 element decreased promoter activity by 10- to 20-fold. Expressing TraR in either host had no effect on activity from any of the four constructs (Table 1 and data not shown). Furthermore, expression from pZLBox3 and pZLBet1 was not affected by addition of AAI (Table 1). However, addition of the signal resulted in a significant decrease in promoter activity from pPBL1 in which the tra box is centered over the −10 element. Repression of the reporter in pPBL1 depends on both TraR and AAI; eliminating one or the other yielded constitutive levels of β-galactosidase activity (Table 1).
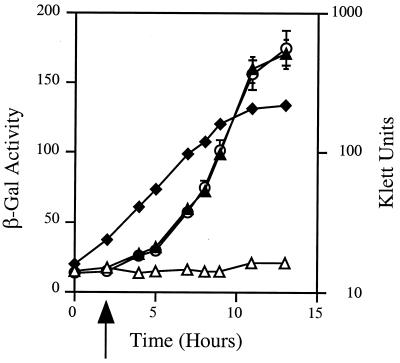
We examined the dependence of repressor activity on the signal by adding AAI to a growing culture and measuring the kinetics of repression. In the absence of AAI, β-galactosidase activity continued to rise throughout the growth of the culture (Fig. 1). However, addition of AAI to the culture at low density led to an almost immediate repression of the reporter that continued over the entire culture cycle.
Figure 1.
Repression of the tra box promoter of pPBL1 depends on TraR and AAI. E. coli DH5α(pPBL1) harboring pZLQR as a source of TraR was grown in A medium to a density of about 107 clony-forming units/ml. At t = 0, the culture was split into three subcultures. IPTG (100 μM) was added to two subcultures to induce expression of traR (○, ▵). After 2 hours of incubation (arrow), AAI (25 nM) was added to the third subculture (▴) and to one of the two subcultures previously induced with IPTG (▵). Incubation was continued, growth was followed by using Klett colorimetry (♦), and samples were taken from each culture at the times indicated and assayed for β-galactosidase activity.
Repression by TraR Shows Specificity for AAI.
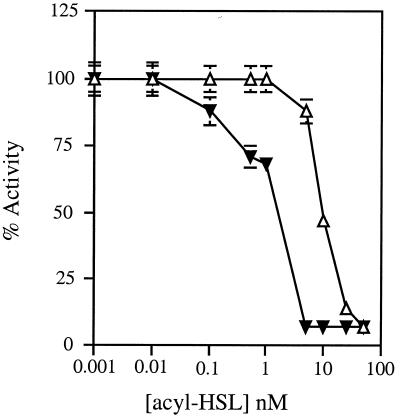
As an activator, TraR responds to AAI at concentrations as low as 0.1 nM (3). It will respond to other acyl-HSLs but requires substantially higher amounts of the alternative signals (3). We assessed signal specificity for the repressor activity of TraR by comparing the concentration dependence of AAI with that of the V. fischeri signal, VAI. Repression mediated by TraR was undetectable at AAI concentrations below 10 pM, and showed half-maximal activity at a concentration of 1 nM (Fig. 2). These levels are similar to those required by TraR for activation (ref. 3 and data not shown). VAI also stimulated repression by TraR but only at substantially higher concentrations (Fig. 2).
Figure 2.
Repression by TraR depends on the structure and concentration of the acyl-HSL. An early exponential-phase culture of E. coli DH5α(pPBL1) harboring pZLQR in A medium containing IPTG was split into 10 subcultures. To 9 of these, synthetic acyl-HSL (▾, AAI; ▵, VAI) was added to the concentrations indicated, incubation was continued until the cultures reached late exponential phase, and the cells were assayed for β-galactosidase activity. An activity of 100% was determined as the level of β-galactosidase activity in cells not exposed to an acyl-HSL.
Repression by TraR Depends On the Integrity of the tra Box.
We constructed 4-bp insertion and deletion mutations at the BglII site, located at the center of the tra box, of both repressor and activator reporter constructs. The insertion abolished promoter activity in the repressor construct and was not further studied. However, the 4-bp deletion mutation, although not significantly affecting the expression of the reporter, abolished repression of this promoter by TraR (Table 2). Identical mutations in the tra box of a positively regulated promoter–reporter construct abolished activation by TraR (Table 2). We also altered the sequence of the box at two positions while retaining symmetry by changing the T at position 4 to C, and the A at position 15 to G. This alteration had little effect on promoter activity, but completely abolished repression and activation by TraR (Table 2).
Table 2.
Deletion and substitution mutations in the tra box abolish activation and repression by TraR
| Plasmid* | tra box† | β-Galactosidase activity‡
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Activation§
|
Repression¶
|
||||||||
| TraR−
|
TraR+
|
TraR−
|
TraR+
|
||||||
| AAI− | AAI+ | AAI− | AAI+ | AAI− | AAI+ | AAI− | AAI+ | ||
| pZLb251/pPBL1 | ATGTGCAGATCTGCACGT | 1 | 1 | 11 | 392 | 89 | 97 | 86 | 5 |
| pZLbm4-15/pLMB4-15 | ATGCGCAGATCTGCGCGT | 3 | 1 | 2 | 2 | 104 | 98 | 96 | 101 |
| pZLb251-D/pPBL1-D | ATGTGCATGCACGT | 1 | 1 | 1 | 1 | 232 | 198 | 221 | 187 |
Each plasmid pair represents the constructs used for the activation and repression assays, respectively.
Underlined nucleotides in the tra box sequence identify the BglII site in its wild-type and deleted forms or the symmetrical base substitutions at the 4 and 15 positions.
Expressed as units per 108 colony-forming units. All values are the averages of three independent experiments with variations <10%.
Assessed in A. tumefaciens.
Assessed in E. coli DH5α.
Repression and Activation Depend on an Intact HTH Domain.
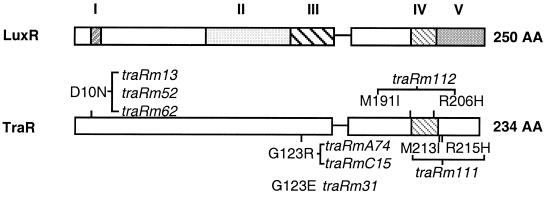
The C-terminal region of TraR contains a HTH motif which in LuxR is essential for activation (6) and also for DNA binding (27, 28). We isolated two mutants, traRm111 and traRm112, that exerted strong dominant negativity against reporter gene activation by wild-type TraR but which, by themselves, failed to activate or repress our reporters (Table 3). Both alleles contain two substitution mutations located at the HTH motif (Fig. 3); in traRm111, Met-213 is replaced by an Ile, and Arg-215 is changed to His, whereas in traRm112, Met-191 is changed to Ile, and Arg-206 is changed to His.
Table 3.
Activation and repression properties of traR mutants
| Allele of traR | Mutation | β-Galactosidase activity*
|
|||||
|---|---|---|---|---|---|---|---|
| Activation†
|
Repression‡
|
Dominant negativity§
|
|||||
| Activity | -Fold activation | Activity | -Fold repression | Activity | -Fold inhibition | ||
| none | — | 2¶ | — | 156 | — | 213 | — |
| wild-type | none | 268 | 134 | 13 | 12 | 267 | — |
| traRm111 | M213I; R215H | 1 | none | 158 | none | 9 | 24 |
| traRm112 | M191I; R206H | 1 | none | 187 | none | 8 | 27 |
| traRΔC-2 | Δ232-T | 1 | none | 155 | none | 5 | 43 |
| traRΔC-4 | Δ230-T | 1 | none | 145 | none | 7 | 30 |
| traRΔC-6 | Δ228-T | 1 | none | 154 | none | 7 | 30 |
| traRΔC-8 | Δ226-T | 2 | none | 144 | none | 6 | 36 |
| traRΔC-10 | Δ224-T | 1 | none | 136 | none | 8 | 27 |
| traRΔC-12 | Δ222-T | 1 | none | 151 | none | 7 | 30 |
| traRΔN2-4 | Δ2-4 | 1 | none | 156 | none | 243 | none |
| traRΔN2-9 | Δ2-9 | 1 | none | 151 | none | 255 | none |
| traRΔN2-49 | Δ2-49 | 1 | none | 140 | none | 237 | none |
| traRm13 | D10N | 2 | none | 13 | 12 | 7 | 30 |
| traRmA74 | G123R | 3 | none | 16 | 10 | 4 | 53 |
| traRm31 | G123E | 31 | 15 | 14 | 11 | 27 | 8 |
Expressed as units per 109 colony-forming units.
Measured as the activation of a traG∷lacZ reporter in NTL4(pH4I41) in the presence of 25 nM AAI.
Measured as the repression of the expression of the promoter–tra box–lacZ complex in DH5α(pPBL1) in the presence of 25 nM AAI.
Assessed in A. tumefaciens harboring clones of pZLQ expressing the mutant alleles of traR as well as the reporter clone pRKLH4I41 that contains a traG∷lacZ fusion and a copy of wild-type traR. AAI was supplied at a concentration of 25 nM and traR on pZLQ was induced by addition of IPTG at 100 μM.
All values are averages of three independent experiments with variation <10%.
Figure 3.
Structural domains of quorum-sensing activators. Domains of LuxR, identified by using genetic analysis and conserved motif structure are: I, autorepression; II, VAI-binding; III, multimerization; IV, DNA-binding (HTH); V, activation. The HTH domain of LuxR and TraR is indicated by the hatched box. The locations and amino acid substitutions in the DNA-binding and positive-control mutants are shown and are followed by the allele names.
N- and C-Terminal Deletions in TraR Abolish Activator and Repressor Activity.
We assessed the activator and repressor properties of TraR mutants truncated for 2–12 C-terminal amino acids. Each failed to activate and to repress expression from the respective reporter plasmids (Table 3). All mutants produced stable protein as assessed by Western analysis (data not shown). Furthermore, each mutant exerted strong dominant negativity against wild-type TraR (Table 3).
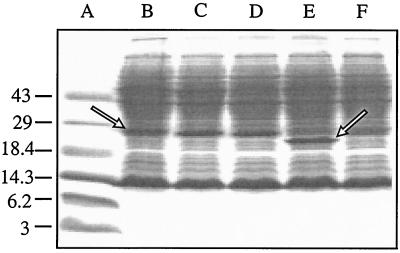
We also constructed TraR mutants deleted for 4, 9, and 49 residues at the amino end. Each deletion allele failed to activate or repress the respective reporter constructs (Table 3). Moreover, when coexpressed with the wild-type gene, none of these mutants affected activation by TraR (Table 3). Truncated proteins with the expected sizes were detected by SDS/PAGE analysis in lysates of cells expressing these alleles (Fig. 4).
Figure 4.
Detection of N-terminal deletion mutants of TraR. Total protein produced by strains harboring clones of traR and its 5′ deletion mutants was prepared, electrophoresed in SDS/polyacrylamide gels, and stained with Coomassie blue as described in Materials and Methods. Lanes contain marker proteins with indicated sizes (kDa) (A) and lysates containing wild-type TraR (B); TraRΔ2–4 (C); TraRΔ2–9 (D); TraRΔ2–49 (E); and no TraR (F).
Mutations in the N-Terminal Half of TraR Affect Activation but Not Repression.
We developed a screen for mutations in TraR that affect activation but not repression. A clone of traR, treated with hydroxylamine in vitro, was electroporated into A. tumefaciens harboring pH4I41, which reports activation by TraR (29). White colonies appearing on screening plates containing AAI and X-Gal were collected and pooled, total plasmid DNA was prepared, and transformed into E. coli DH5α harboring pPBL1, which reports AAI-dependent repression by TraR. White colonies appearing on the screening plates harbor alleles of traR that are mutant for activation but retain repressor activity. Six such positive-control mutants were obtained, each from an independent screen. In all six cases, when recloned, the positive-control phenotype transferred with the gene. The mutants fell into two classes (Fig. 3). In one class, Asp-10 is changed to Asn. In the second, Gly-123 is changed to either Arg or Glu. Western analysis indicated that each mutant produces full-sized protein at levels indistinguishable from wild-type TraR (data not shown).
Whereas the three unique mutants retain full repressor activity, the D10N and the G123R mutants fail to activate expression, while the G123E mutant retains some AAI-dependent activator activity (Table 3). As a repressor, the G123R mutant exhibits a concentration dependency for AAI indistinguishable from that of wild-type TraR (data not shown). All three mutants strongly inhibited TraR-mediated activation when coexpressed with the wild-type allele (Table 3).
DISCUSSION
Positioning the tra box over the −10 element results in a promoter that is strongly repressible by TraR. We propose that repression results entirely from TraR binding at the tra box, and consequently, that this activity can be used as an in vivo measure of DNA binding by the protein. In support of this, repression depends on the presence and also the fidelity of the tra box; mutations within the inverted repeat that abolish activation of a positively regulated promoter also abolish repression of the chimeric promoter. Moreover, mutants of TraR with substitutions within or near the HTH motif fail to repress and also fail to activate. In these respects, TraR resembles LuxR and LasR. In both, alterations in their respective target sites abolish activation (30, 31). Similarly, mutations in the HTH region of LuxR negatively affect activation, presumably by interfering with DNA binding (4, 8).
The location of the tra box within the promoter is critical for repressor activity. Failure to repress promoters containing the box between the −10 and the −35 elements or downstream of the −10 element may be due to improper phasing. Alternatively, the promoter we used is extremely active, reflecting a strong affinity for RNA polymerase. It is possible that TraR does not bind the tra box in these two constructs with sufficient affinity to prevent interaction between the promoter and RNA polymerase. Consistent with this possibility, the only construct repressible by TraR contains the tra box centered over the −10 element, a location that weakens considerably the activity of the promoter itself.
Repression by TraR, like activation, depends on AAI. That TraR shows the same requirements in the two modes indicates that the protein–acyl-HSL–DNA interactions are similar in both systems. We conclude from this that TraR cannot interact with the tra box in the absence of AAI. This is consistent with the hypothesis, developed for LuxR, that in the absence of the acyl-HSL, an N-terminal portion of the activator occludes the C-terminal DNA-binding domain (6, 32).
Deleting as few as 2 amino acids from the C terminus of TraR abolishes both repression and activation, indicating that this region of the protein is essential for DNA binding and possibly also for transcriptional activation. These short deletions do not extend into the HTH motif, suggesting that components of the protein other than this domain are essential for these activities. In contrast, removing from 10 to 40 amino acids from the C terminus of the somewhat longer LuxR protein abolishes activation but not autorepression, suggesting that this region, although essential for activation, is not required for DNA binding (10).
Two sets of results indicate that the N-terminal half of TraR is required for activation. First, removing as few as 4 residues from this end of the protein abolished both repressor and activator activities. Moreover, despite considerable effort, we were not able to isolate N-terminal deletion mutants of TraR with properties like those of LuxRΔN. Even derivatives of TraR truncated to precisely those lengths which, in LuxR result in an acyl-HSL-independent activator (6), lost both repressor and activator activities (data not shown). Second, the two classes of positive control mutants harbor substitutions located in the N-terminal half of the protein. No other such alleles were isolated, suggesting that we have identified the two positions in this region of the protein most critical for activator activity. Asp-10 is not conserved in any other member of the LuxR family, although all contain one or more Asp residues within the first 18 positions (33, 34). On the other hand, Gly-123 is conserved in many members of the family (33, 34). This residue is located close to the C-terminal end of the proposed acyl-HSL-binding region defined for LuxR (7, 8). Repression by these mutants depends on AAI. Moreover, the concentration-dependency profile for repression exhibited by the G123R mutant is indistinguishable from that of wild-type TraR (data not shown), ruling out the possibility that Gly-123 is necessary for binding the signal. Whereas the G → R substitution completely abolished activation, the G123E mutant retains some activator activity. Thus, only the activator properties of the protein depend on the nature of the residue at position 123. These results suggest that Gly-123 is required for a structural feature of TraR associated with activation but not with signal binding or for subsequent interaction with DNA. Although no such mutants of LuxR have been described, positive-control mutants of other activators, including AraC, FIS, and CAP of E. coli have been analyzed in some detail (35–37). Interestingly, some such mutants of FIS and CAP contain substitutions at glycines near or within domains known to interact with RNA polymerase (38, 39).
Like the analogous mutants of LuxR (10), our C-terminal deletion mutants are strongly dominant over the wild-type activator. Such dominant negativity is consistent with a model in which TraR, like LuxR, forms homomultimers. On the other hand, mutants of TraR with N-terminal deletions as short as 4 residues neither activate nor repress transcription and do not inhibit wild-type TraR. Similarly, N-terminal deletion mutants of LuxR are recessive to the wild-type activator (6). The absence of dominant negativity suggests that these deletion derivatives cannot interact with wild-type TraR. Alternatively, these mutant proteins may interact with TraR to form functional, AAI-dependent heteromeric activators.
Although members of the same family, TraR and LuxR differ in several respects. First, in contrast to LuxR, the extreme C and N termini of TraR are required for DNA binding, as well as for transcriptional activation. Second, autorepression is not dependent on VAI when LuxR is expressed at high levels (10). In contrast, repression by TraR depends on AAI even when the protein is overexpressed. Finally, the isolation of positive control mutants with substitutions in the N-terminal half of TraR suggests that this portion of the protein plays a role in transcriptional activation. Such a function is difficult to reconcile with the properties of LuxRΔN. This mutant, which lacks residues 2–162 (6), strongly activates VAI-independent expression of the lux operon, suggesting that the N-terminal domain is not required for DNA binding or transcriptional initiation. It is possible that TraR and LuxR activate transcription by different mechanisms. This possibility may explain our inability to isolate LuxRΔN-like mutants of TraR. Alternatively, LuxRΔN and wild-type LuxR themselves may activate transcription by somewhat different mechanisms (32). Despite these differences, our data support a model in which DNA binding at specific target sites by TraR and by analogy, most other activators of the LuxR family, depends on interaction with their acyl-HSL signals. The structural alterations that occur in these proteins as a result of binding the signal remain to be determined. However, our recent results indicate that dimerization of TraR requires AAI (A. J. Smyth, Y. P. Qin, and S.K.F., unpublished data).
Acknowledgments
We thank Stanley Maloy and Jeffery Gardner for helpful discussions. This work was supported by Grant R01 GM52465 from the National Institutes of Health to S.K.F.
ABBREVIATIONS
- acyl-HSL
acyl-homoserine lactone
- AAI
Agrobacterium autoinducer
- HTH
helix–turn–helix
- IPTG
isopropyl-β-d-thiogalactopyranoside
- VAI
Vibrio autoinducer
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactopyranoside
References
- 1.Piper K R, Beck von Bodman S, Farrand S K. Nature (London) 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua W C, Winans S C. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Murphy P J, Kerr A, Tate M E. Nature (London) 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]
- 4.Devine J H, Contryman C, Baldwin T O. Biochemistry. 1988;27:837–842. [Google Scholar]
- 5.Eberhard A, Burlingame A L, Eberhard C, Nealson K H, Oppenheimer N J. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 6.Choi S H, Greenberg E P. Proc Natl Acad Sci USA. 1991;88:11115–11119. doi: 10.1073/pnas.88.24.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slock J, VanRiet D, Kolibachuk D, Greenberg E P. J Bacteriol. 1990;172:3979. doi: 10.1128/jb.172.7.3974-3979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shadel G S, Young R, Baldwin T O. J Bacteriol. 1990;172:3980–3987. doi: 10.1128/jb.172.7.3980-3987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi S H, Greenberg E P. Mol Marine Biol Biotechnol. 1992;1:408–413. [Google Scholar]
- 10.Choi S H, Greenberg E P. J Bacteriol. 1992;174:4064–4069. doi: 10.1128/jb.174.12.4064-4069.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrand S K, Hwang I, Cook D M. J Bacteriol. 1996;178:4233–4247. doi: 10.1128/jb.178.14.4233-4247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang I, Li P-L, Zhang L, Piper K R, Cook D M, Tate M E, Farrand S K. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuqua C, Winans S C. J Bacteriol. 1996;178:435–440. doi: 10.1128/jb.178.2.435-440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Winans S C. Proc Natl Acad Sci USA. 1999;96:4832–4837. doi: 10.1073/pnas.96.9.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunlap P V, Ray J M. J Bacteriol. 1989;171:3549–3552. doi: 10.1128/jb.171.6.3549-3552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shadel G S, Baldwin T O. J Bacteriol. 1991;173:568–574. doi: 10.1128/jb.173.2.568-574.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 18.Cangelosi G A, Best E A, Martinetti G, Nester E W. Methods Enzymol. 1991;204:384–397. doi: 10.1016/0076-6879(91)04020-o. [DOI] [PubMed] [Google Scholar]
- 19.Chilton M-D, Currier T C, Farrand S K, Bendich A J, Gordon M P, Nester E W. Proc Natl Acad Sci USA. 1974;71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller J. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 21.Luo Z-Q, Farrand S K. J Bacteriol. 1999;181:618–626. doi: 10.1128/jb.181.2.618-626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw P D, Ping G, Daly S L, Cha C, Cronan J E, Jr, Rinehart K L, Farrand S K. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Eede G, Deblaere R, Goethals K, van Montagu M, Holsters M. Mol Plant Microbe Interact. 1992;5:228–234. doi: 10.1094/mpmi-5-228. [DOI] [PubMed] [Google Scholar]
- 24.Maloy S, Youderian P. In: Methods in Molecular Genetics. Comley I, editor. Vol. 3. New York: Academic; 1994. pp. 205–233. [Google Scholar]
- 25.Kovach M E, Elzer P H J, Hill D S, Roberston G T, Farris M A, Roop I, Peterson K M. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 26.Hwang I, Farrand S K. Appl Environ Microbiol. 1994;60:913–920. doi: 10.1128/aem.60.3.913-920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens A M, Dolan K M, Greenberg E P. Proc Natl Acad Sci USA. 1994;91:12619–12623. doi: 10.1073/pnas.91.26.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens A M, Greenberg E P. J Bacteriol. 1997;179:557–562. doi: 10.1128/jb.179.2.557-562.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang I, Cook D M, Farrand S K. J Bacteriol. 1995;177:449–458. doi: 10.1128/jb.177.2.449-458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devine J H, Shadel G, S, Baldwin T O. Proc Natl Acad Sci USA. 1989;86:5688–5692. doi: 10.1073/pnas.86.15.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rust L, Pesci E C, Iglewski B H. J Bacteriol. 1996;178:1134–1140. doi: 10.1128/jb.178.4.1134-1140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuqua C, Greenberg E P. Curr Opin Microbiol. 1998;1:183–189. doi: 10.1016/s1369-5274(98)80009-x. [DOI] [PubMed] [Google Scholar]
- 33.Fuqua C, Winans S C, Greenberg E P. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 34.Sitnikov D M, Schineller J B, Baldwin T O. Mol Microbiol. 1995;17:801–812. doi: 10.1111/j.1365-2958.1995.mmi_17050801.x. [DOI] [PubMed] [Google Scholar]
- 35.Saviola B, Seabold R R, Schleif R F. J Bacteriol. 1998;180:4227–4232. doi: 10.1128/jb.180.16.4227-4232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gosink K K, Gaal T, Bokal A J, Gourse R L. J Bacteriol. 1996;178:5182–5187. doi: 10.1128/jb.178.17.5182-5187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Zhang X, Ebright R H. Proc Natl Acad Sci USA. 1993;90:6081–6085. doi: 10.1073/pnas.90.13.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bokal A J, Ross W, Gourse R L. J Mol Biol. 1995;245:197–207. doi: 10.1006/jmbi.1994.0016. [DOI] [PubMed] [Google Scholar]
- 39.Niu W, Zhou Y, Dong Q, Ebright Y W, Ebright R H. J Mol Biol. 1994;243:595–602. doi: 10.1016/0022-2836(94)90034-5. [DOI] [PubMed] [Google Scholar]