Abstract
Aim: To study the relation between visual impairment and ability to care for oneself or a dependant in older people with age related macular degeneration (AMD).
Method: Cross sectional study of older people with visual impairment due to AMD in a specialised retinal service clinic. 199 subjects who underwent visual function assessment (fully corrected distance and near acuity and contrast sensitivity in both eyes), followed by completion of a package of questionnaires dealing with general health status (SF36), visual functioning (Daily Living Tasks Dependent on Vision, DLTV) and ability to care for self or provide care to others. The outcome measure was self reported ability to care for self and others. Three levels of self reported ability to care were identified—inability to care for self (level 1), ability to care for self but not others (level 2), and ability to care for self and others (level 3).
Results: People who reported good general health status and visual functioning (that is, had high scores on SF36 and DLTV) were more likely to state that they were able to care for self and others. Similarly people with good vision in the better seeing eye were more likely to report ability to care for self and others. People with a distance visual acuity (DVA) worse than 0.4 logMAR (Snellen 6/15) had less than 50% probability of assigning themselves to care level 3 and those with DVA worse than 1.0 logMAR (Snellen 6/60) had a probability of greater than 50% or for assigning themselves to care level 1. Regression analyses with level of care as the dependent variable and demographic factors, DLTV subscales, and SF36 dimensions as the explanatory variables confirmed that the DLTV subscale 1 was the most important variable in the transition from care level 3 to care level 2. The regression analyses also confirmed that the DLTV subscale 2 was the most important in the transition from care level 3 to care level 1.
Conclusions: Ability to care for self and dependants has a strong relation with self reported visual functioning and quality of life and is adversely influenced by visual impairment. The acuity at which the balance of probability shifts in the direction of diminished ability to care for self or others is lower than the level set by social care agencies for provision of support. These findings have implications for those involved with visual rehabilitation and for studies of the cost effectiveness of interventions in AMD.
Keywords: age related macular degeneration, older adults, quality of life, self care, visual impairment
The burgeoning elderly population in Western societies is a matter of some concern. The Medical Research Council (MRC) Cognitive Function in Ageing Study (CFAS) showed that 11% of men and 19% of women of age 65 and over had some form of disability with limitations in daily living activities and, of those residing in private households, one third were dependent on substantial support from formal services.1 Cognitive and sensory disabilities in the elderly are common2,3 and irreversible severe vision loss in older people is most often caused by age related macular degeneration (AMD).4,5 It is widely accepted that AMD will increase in prevalence because of extended life expectancy, and consequently will have a major impact on public health and other services.6–8 Several studies in the United States have shown that vision impairment has a significant and negative effect on the emotional and physical wellbeing and independence of older people.9–11 However, the relation between the level of visual impairment and independent living or the ability of the visually impaired to provide care for dependants has never been explored. We compiled a clinical database of subjects with AMD who completed questionnaires on visual functioning and health related quality of life. In a short series of questions we asked respondents to provide us with their perception of how their visual impairment impacted on their ability to care for self or provide care to others. The present report is a summary of the findings from this group of patients.
METHODS
Patients with a diagnosis of AMD who were attendees at a specialist macular clinic between March 1997 and September 1999 were requested to complete a set of questionnaires. Ethical approval was obtained before compiling the clinical database and the studies were carried out in accordance with the tenets of the Declaration of Helsinki on research in human volunteers. Inclusion criteria were broad and patients with a diagnosis of exudative or dry AMD (age above 50 years) of either sex were requested to participate. We did not seek to exclude patients with severe levels of visual impairment or poor general health. Of the 215 patients who were approached all but five gave informed consent to be interviewed. The sampling method was opportunistic and was not subject to a formal sample size calculation.
The package of instruments consisted of (a) the SF36 a generic health status instrument,12 (b) The Daily Living Tasks Dependent on Vision (DLTV) a visual functioning index,13 and (c) a short list of questions which asked about ability to care for self and others. The SF36 is a well known generic multiattribute, multidimensional health status questionnaire that has been extensively used in a variety of age groups and disease conditions.12,14 The DLTV is an instrument which was specifically designed to assess performance of everyday tasks by older people with irreversible visual impairment in one or both eyes.13,15,16 Details of the content of the DLTV and its scoring have already been published and it has been tested and validated in AMD sufferers.13,14,16 Briefly, the DLTV consists of four subscales and the items comprising each of these are shown in table 1. DLTV items are scored on a scale of 1 to 4 with 1 representing inability and 4 perfect ability to undertake the task. The items within each subscale are summed and this score is transformed into a scale of 0 to 100 where 0 represents inability to function and 100 perfect function. The third instrument consisted of a list of four simple questions. Two of the questions dealt with accidental injuries in the preceding 3 months and any treatments given if an injury had been sustained. The next two questions asked the respondent whether eyesight had prevented them from attending to their own needs or those of others (table 2).
Table 1.
Items in the daily living tasks dependent on vision by dimension
| Subscale 1 (8 items) |
| Reading normal size newsprint |
| Reading correspondence—eg, letters and bills |
| Signing documents—eg, cheques |
| Identifying money from a wallet |
| Reading road signs/street names |
| Watching TV programmes |
| Distinguishing a person’s features across the street |
| Distinguishing a person’s features across the room |
| Subscale 2 (8 items) |
| Distinguishing a person’s features at arm’s length |
| Reading newspaper headlines |
| Pouring yourself a drink |
| Using kitchen appliances |
| Recognising seasonal changes in the garden |
| Cutting up food on your plate |
| Enjoying the scenery if out for a drive |
| Cutting fingernails |
| Subscale 3 (7 items) |
| How would you rate overall near vision? |
| How would you rate overall distance vision? |
| Confidence in ability to walk around one’s own neighbourhood |
| Confidence in an ability to walk around an unfamiliar neighbourhood |
| Do you agree? I feel I have to be more careful because of my eye condition |
| Noticing objects off to either side |
| Seeing steps and using them |
| Subscale 4 (2 items) |
| Adjusting to brightness after being in the dark |
| Adjusting to darkness after being in the light |
Table 2.
Items in the short questionnaire on self reported ability to care for self and others and accidental injuries
| Item | Yes | No | |
| 1 | In the past 3 months have you had any accidents? | ||
| Specify type of accident | |||
| 2 | In the past 3 months have you had any treatment for an accidental injury? | ||
| Specify treatment | |||
| 3 | Does your eyesight prevent you from attending to the needs of a spouse, relative or friend? | ||
| 4 | Does your eyesight prevent you from attending to your own needs? |
Distance visual acuity
All subjects underwent visual acuity testing according to a structured protocol. Briefly, a full refraction was performed following which best corrected DVA was obtained using retroilluminated ETDRS charts at a testing distance of 4 metres. If the subject was unable to read a minimum of 20 letters at the 4 metre distance, the testing was repeated with the chart placed at 1 metre.
Statistical analyses
The Statistical Package for Social Sciences (SPSS) was used to record, retrieve, and analyse data. Measures of vision recorded on each eye and the responses to all of the questionnaires entered into a spreadsheet.
Summary statistics were generated to describe the visual characteristics of the group. The relation between accidental injury and clinical measures of vision was tested using t tests. Mean scores were generated for each of the eight dimensions of the SF36 and the four subscales of the DLTV. Subjects were assigned to three care levels based on their responses to items which asked about ability to care for self or provide care to others. The categories were could not care for self (level 1), could not care for others but could care for self (level 2), and could care for both self or others (level 3). Analysis of variance was used to examine relations between DLTV subscales, SF36 dimensions, and clinical measures of vision.
In order to establish the acuity levels at which the balance of probability shifts from one care level to the next we used multinomial logistic regression with care level as the outcome variable and DVA as the sole explanatory variable.
Multinomial logistic regression was used to estimate relations between the three levels of care and a number of explanatory variables which included age and sex, distance acuity in the better eye, DLTV subscales, and SF36 dimensions. We then entered only those variables with a p value below 0.10 and were not counterintuitive in the direction of their effects, into binomial logistic regression models to confirm the relations detected by the multinomial model. This involved testing of the base category (care level 3 which had the largest number of subjects) with each of the other categories (care level 2 and care level 1) in turn and employed a stepwise backwards Wald procedure to identify the most significant variables of the six included (age, the physical functioning and mental health dimensions of the SF36, domains 1 and 2 of the DLTV, and visual acuity in the better eye).
RESULTS
Subjects
A total of 215 subjects were approached and 210 consented to take part. Data from both clinical assessments and questionnaire administration were available on 199 subjects. Eleven subjects (four men and seven women) did not complete all three questionnaires at the clinic visit owing to time constraints and are excluded from the analysis. In six subjects some questionnaire fields were incomplete but data from these subjects were included in this analysis. There were 74 men and 125 women with ages ranging from 50 to 97 years with a mean of 74 (SD 9). Approximately one third of the sample (78) were classified as visually unimpaired—that is, with an acuity of logMAR 0.2 (6/9 Snellen equivalent) or better in the better seeing eye and the remainder were bilaterally visually impaired. There were no differences in age or sex distribution between the 199 in whom questionnaire data were complete and the 11 who did not complete the full package of questionnaires.
Clinical measures of vision
The mean levels of distance and near acuity and contrast sensitivity in the better and worse eyes of the 199 eligible subjects are shown in table 3.
Table 3.
Clinical measures of vision in better and worse eyes
| DVA (199) | NVA (199) | CS (195) | |
| Better eyes | 0.47 (0.42) | 0.70 (0.48) | 1.17 (0.40) |
| Worse eyes | 1.08 (0.52) | 1.30 (0.43) | 0.60 (0.53) |
DVA = distance visual acuity, NVA = near visual acuity, CS = contrast sensitivity.
Mean (SD) logMAR distance and near acuity and log contrast sensitivity.
Only 16 subjects reported an accidental injury in the 3 months preceding questionnaire administration. Commonly cited injuries included burns, scalds, sprains, and falls. Average measures of vision were worse in those subjects reporting accidental injuries than those who did not and these differences were statistically significant with NVA (p<0.05), borderline significance for DVA (p = 0.07), and not significant for CS, using independent samples t tests.
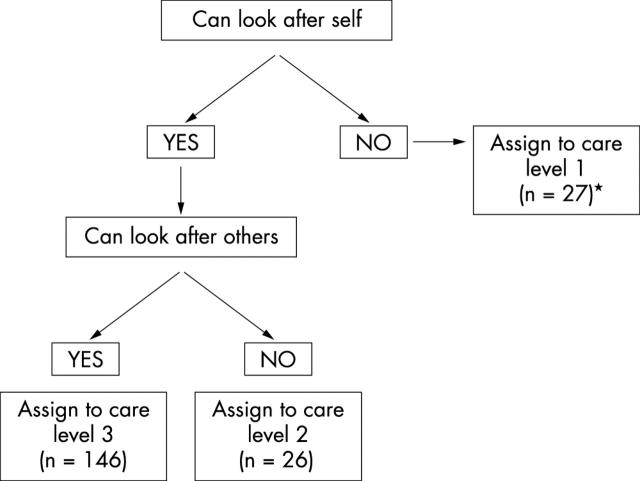
Figure 1 shows the assignment of subjects to the different care levels based on ability to care for self and others. Only one subject reported ability to care for others but inability to care for self. This subject was assigned to care level 1 (that is inability to care for self or others) for the purposes of this analysis.
Figure 1.
Schematic for the allocation of subjects to the different care levels (n = 199). *One subject who was unable to care for self reported ability to care for others. This subject was assigned to care level 1 (making the size of group 27).
A clear relation was noted between the three levels of care and each of the clinical measures of vision in both better and worse eyes (table 4) with care level 3 having best vision and care level 1 worst vision. While all comparisons were statistically significant (by one way ANOVA) those with the worse seeing eye were less striking than those involving the better seeing eye.
Table 4.
Clinical measures of vision in better and worse eyes by level of care category
| Clinical measures of vision better eyes (standard deviation) | Clinical measures of vision in worse eyes (standard deviation) | |||||
| DVA | NVA | Contrast | DVA | NVA | Contrast | |
| Level 1: Cannot care for self (27) | 0.88 (0.43) | 1.12 (0.44) | 0.83 (0.50) | 1.35 (0.41) | 1.46 (0.30) | 0.34 (0.43) |
| Level 2: Can look after self but not others (26) | 0.70 (0.40) | 0.94 (0.39) | 1.03 (0.35) | 1.28 (0.49) | 1.45 (0.31) | 0.43 (0.50) |
| Level 3: Can care for self and others (146) | 0.35 (0.36) | 0.57 (0.44) | 1.26 (0.35) | 1.0 (0.52) | 1.24 (0.45) | 0.67 (0.54) |
| One way ANOVA | p<0.001 | p<0.001 | p<0.001 | p<0.01 | p<0.001 | p<0.01 |
DVA = distance visual acuity, NVA = near visual acuity, CS = contrast sensitivity.
The probability of reporting inability to care for self and or others was examined (table 5). To have a posterior probability of 0.5 or greater for being placed in care level 3, DVA was required to be better than 0.5 logMAR. Similarly, to have a probability of 0.5 or greater for being placed in care level 1, DVA was required to be worse than 1.0 logMAR. The most likely DVA at which placement in care level 2 occurred was around 0.7 logMAR.
Table 5.
Posterior probabilities for assignment to level of care categories based on DVA in the better seeing eye
| DVA in better eye | Probability of being assigned to care level 1 | Probability of being assigned to care level 2 | Probability of being assigned to care level 3 |
| −0.2 | 0.04 | 0.14 | 0.83 |
| −0.1 | 0.05 | 0.16 | 0.79 |
| 0.0 | 0.07 | 0.19 | 0.74 |
| 0.1 | 0.09 | 0.22 | 0.69 |
| 0.2 | 0.12 | 0.25 | 0.63 |
| 0.3 | 0.15 | 0.28 | 0.57 |
| 0.4 | 0.19 | 0.31 | 0.50 |
| 0.5 | 0.23 | 0.34 | 0.43 |
| 0.6 | 0.28 | 0.35 | 0.37 |
| 0.7 | 0.33 | 0.37 | 0.31 |
| 0.8 | 0.38 | 0.37 | 0.25 |
| 0.9 | 0.43 | 0.37 | 0.20 |
| 1.0 | 0.48 | 0.37 | 0.16 |
| 1.1 | 0.52 | 0.36 | 0.12 |
| 1.2 | 0.57 | 0.34 | 0.09 |
| 1.3 | 0.61 | 0.32 | 0.07 |
| 1.4 | 0.65 | 0.30 | 0.05 |
| 1.5 | 0.68 | 0.28 | 0.04 |
| 1.6 | 0.71 | 0.26 | 0.03 |
| 1.7 | 0.74 | 0.24 | 0.02 |
This table shows that as DVA worsens the probability of being assigned to care level 1 increases while that of assignment to care level 3 diminishes. The maximum probability of being assigned to care level 2 occurs when DVA is around 0.7 logMAR.
All DLTV domains were associated with ability to care for self or others, with three of the four subscales demonstrating highly significant relations by one way ANOVA (table 6). On examination of the associations between the SF36 and ability to care for self or others, the physical functioning (PF) dimension exhibited a statistically highly significant relation (p<0.001) with the levels of care by one way ANOVA. PF scores were highest in self reported care level 3, lowest in care level 1, and intermediate in care level 2 (table 7). Four other dimensions of the SF36—namely, pain, mental health, general health perception, and energy and vitality exhibited less strong but significant relations with levels of care (table 7).
Table 6.
DLTV subscales and levels of care
| DLTV subscale | Subscale 1 (resolution items) | Subscale 2 (complex visual tasks) | Subscale 3 (confidence related items) | Subscale 4 (light and dark adaptation) |
| Level 1: Cannot care for self (27) | 18 (22) | 41 (24) | 27 (15) | 47 (31) |
| Level 2: Can look after self but not others (26) | 27 (25) | 60 (22) | 37 (19) | 64 (28) |
| Level 3: Can care for self and others (146) | 61 (32) | 82 (22) | 58 (22) | 68 (26) |
| One way ANOVA | p<0.001 | p<0.001 | p<0.001 | p<0.01 |
DLTV = daily living tasks dependent on vision. Marked differences in mean subscale scores are seen between the care levels in subscales 1 and 2.
Table 7.
Relations between SF36 dimension scores and level of care categories
| PF | RP | RE | P | SF | MH | EV | GHP | |
| Level 1: Cannot care for self (27) | 37.7 (30.4) | 65.4 (45.3) | 76.9 (40.8) | 65.4 (31.0) | 82.7 (31.4) | 62.2 (21.9) | 49.4 (20.2) | 57.6 (21.4) |
| Level 2: Can look after self but not others (26) | 53.2 (31.5) | 73.1 (45.2) | 65.4 (48.5) | 83.8 (25.4) | 85.6 (25.4) | 66.2 (25.7) | 53.3 (23.2) | 65.2 (23.5) |
| Level 3: Can care for self and others (146) | 67.8 (25.2) | 75.0 (40.2) | 76.1 (41.7) | 81.0 (24.5) | 90.2 (20.0) | 73.3 (20.9) | 59.9 (21.6) | 72.2 (19.9) |
| ANOVA | p<0.001 | p>0.05 | p>0.05 | p<0.05 | p>0.05 | p<0.05 | p<0.05 | p<0.01 |
PF = physical functioning, RP = role physical, RE = role emotional, P = pain, SF = social functioning, MH = mental health, EV = energy and vitality, GHP = general health perception.
Multinomial logistic regression was performed with level of care as the dependent variable and the following explanatory variables (age, sex, all four DLTV subscales, the five SF36 dimensions which were significantly associated with care levels on univariate analysis and visual acuity in the better eye). The model (table 8A) showed that only six of the entire selection of explanatory variables passed our initial selection criteria (p value not exceeding 0.10 and direction of the effect intuitively correct). These were age, the physical functioning and mental health dimensions of the SF36, subscales 1 and 2 of the DLTV and distance acuity in the better seeing eye.
Table 8.
Model of logistic regression
| A Multinomial regression | |||||
| Self reported level of care | Explanatory variables | Exp(B) | Wald | Significance | 95% CI |
| Level 2 | Age | 1.008 | 0.05 | 0.82 | 0.942 to 1.078 |
| PF | 0.982 | 1.85 | 0.17 | 0.956 to 1.008 | |
| P | 1.013 | 1.05 | 0.31 | 0.988 to 1.039 | |
| MH | 0.984 | 0.82 | 0.37 | 0.951 to 1.019 | |
| EV | 1.012 | 0.28 | 0.60 | 0.968 to 1.057 | |
| GHP | 0.985 | 0.80 | 0.37 | 0.952 to 1.018 | |
| DLTV subscale 1 | 0.962 | 4.08 | 0.044 | 0.927 to 0.999 | |
| DLTV subscale 2 | 1.002 | 0.01 | 0.91 | 0.967 to 1.039 | |
| DLTV subscale 3 | 0.991 | 0.16 | 0.69 | 0.950 to 1.034 | |
| DLTV subscale 4 | 1.020 | 3.42 | 0.064 | 0.999 to 1.041 | |
| DVA better eye | 1.63 | 0.31 | 0.58 | 0.29 to 9.09 | |
| Level 1 | Age | 0.890 | 7.96 | 0.005 | 0.821 to 0.965 |
| PF | 0.940 | 12.58 | 0.000 | 0.909 to 0.973 | |
| P | 0.999 | 0.01 | 0.941 | 0.970 to 1.028 | |
| MH | 0.960 | 3.37 | 0.066 | 0.919 to 1.003 | |
| EV | 1.062 | 4.13 | 0.04 | 1.002 to 1.125 | |
| GHP | 0.992 | 0.14 | 0.703 | 0.973 to 1.025 | |
| DLTV subscale 1 | 0.985 | 8.40 | 0.004 | 0.929 to 1.044 | |
| DLTV subscale 2 | 0.962 | 2.66 | 0.10 | 0.918 to 1.008 | |
| DLTV subscale 3 | 1.009 | 0.09 | 0.76 | 0.954 to 1.067 | |
| DLTV subscale 4 | 0.998 | 0.01 | 0.91 | 0.973 to 1.025 | |
| DVA better eye | 6.65 | 2.80 | 0.09 | 0.72 to 61.1 | |
| B Binomial logistic regression (base category level 3) | |||||
| Level 2 | DLTV subscale 1 | 1.039 | 17.09 | 0.000 | 1.020 to 1.058 |
| SF 36 PF | 1.017 | 3.89 | 0.049 | 1.000 to 1.033 | |
| C Binomial logistic regression (base category level 3) | |||||
| The multinomial regression model (A) was run with the three levels of care as the dependent variable and age, sex, SF36 dimensions, and DLTV subscales as explanatory variables. The model shows that the decrease in scores in DLTV subscale 1 was most strongly influenced by the transition from care level 3 to 2. The decrease in PF scores and age most strongly influenced the transition from care level 2 to level 1 with a contribution from DLTV subscale 2. To progress to a more parsimonious model variables that were counterintuitive were first removed followed by those explanatory variables whose p values exceeded 0.1. (SPSS default for stepwise backwards and also equivalent to a 5% test in one direction only.) | |||||
| The binomial regression model (B) confirmed that DLTV subscale 1 was most important in the transition from care level 3 to 2 while DLTV subscale 2 was most important in the transition to care level 1 (C). | |||||
| Level 1 | DLTV subscale 2 | 1.041 | 9.54 | 0.002 | 1.015 to 1.068 |
| SF 36 PF | 1.031 | 8.37 | 0.004 | 1.010 to 1.053 | |
| Min DVA | 0.24 | 3.15 | 0.076 | 0.05 to 1.16 | |
On testing the transition from care level 3 to care level 2 on a binomial logistic regression model, DLTV 1 followed by the PF dimension of the SF36 were the most important variables (table 8B). On testing the transition from care level 3 to level 1, DLTV 2, the PF dimension of the SF36 and DVA in the better seeing eye were most important. Age was not included in the first model because it was insignificant in the transition from care level 3 to level 2. In the second model which examined transition from care level 3 to level 1, although marginally significant age was excluded because the direction was counterintuitive in that the effect of increasing age was to reduce the probability of being in care level 1. A model for transition from level 2 to level 1 was not run owing to small numbers within these two categories.
DISCUSSION
To the best of our knowledge this is the first study to examine the relation between visual impairment and self reported ability to care for oneself or a dependant. We used multinomial logistic regression followed by binomial regression to model self allocation to the three care categories on the basis of the explanatory variables consisting of demographic factors (age and sex), DVA in the better eye, general health status (SF36 dimensions), and visual functioning (DLTV subscales). Combinations of DLTV subscales 1 and 2 and the PF dimension of the SF36, were selected as factors which explained most of the variation in self allocation to the different care levels. Interestingly, the effect of distance acuity in the better eye was of only marginal importance. While the relation with the SF36 was not surprising it was noteworthy that the binomial regression model suggested that the DLTV distinguished between the different self reported levels of care categories. The DLTV subscale 1, which contained items mainly relating to ability to undertake tasks of visual resolution such as reading, distinguished those subjects who could care for both self and others (care level 3) from those who could only care for self (care level 2). By contrast, subscale 2, which contained items relating to household chores such as cooking, distinguished between those who could care for self and others (care level 3) from those who could not care for self (care level 1).
This study also found that self reported visual functioning and physical functioning were significantly better indicators of ability to care for self or provide care than clinical measures of vision, which are parameters that have been traditionally accepted as markers of visual disability. While these findings suggest that it may be opportune to revisit the routine practice of relying only on distance visual acuity as the primary marker for triggering statutory support services, DVA is a commonly used well understood surrogate for visual function and clearly further studies are needed with larger sample sizes which address the validity of the questions on self care and the sensitivity and specificity of the different cutoff points on the SF36 and the DLTV. Our findings do however question the rationale for the selection of the level of acuity at which registration for visual impairment is currently set (partial sightedness is defined as a vision of logMAR 1.0 in the better eye (Snellen 6/60) and blindness is defined as a vision equal to or worse than logMAR 1.4 (Snellen 3/60 or worse) and lends credibility to the current practice of offering low vision support services at significantly better levels of vision than that at which statutory support is triggered.
This study also confirmed that bilateral visual impairment is associated with a greater tendency to accidental injury and in this regard is concordant with the observations of many previous reports that older visually impaired people are more likely to fall or injure themselves.10,17,18
Although we did not find that clinical measures of vision were the best indicators of self reported ability to care for self or provide care, as DVA is the most commonly used marker for visual function it seemed appropriate to look at its influence on such self reported dependency. On running the logistic regression model with only DVA entered as an explanatory variable, we found that the balance of probability for reporting a shift from care level 3 to level 2 occurred at an acuity of approximately 0.5 logMAR. A further shift from level 2 to care level 1 occurred at acuity of 1.0 logMAR. These findings raise concerns that when acuity is worse than 1.0 logMAR in the better seeing eye, visual impairment of this level of severity may act as a trigger into a dependency state.
Cross sectional studies have identified a high prevalence of visual impairment in nursing home residents suggesting that sensory disability may contribute to loss of independence.19,20 While a general decline in health and increasing physical frailty are recognised as contributors to placement in nursing homes and care facilities,10,21 at present it is not possible to attribute causality between visual impairment and the need for institutionalised care in older people as the high prevalence of co-morbidities in this age group can act as confounding factors. It is therefore noteworthy that the present study while clearly identifying physical functioning as an indicator for the loss of ability to care for self also highlighted a potential contributory role for visual impairment. Although, no information on co-morbidities was collected, the concurrently acquired SF36 data acted as a marker for general health status and were taken into account in the models constructed to examine the relation between visual impairment and independent living. However, the present study is limited by the smaller numbers of subjects reporting inability to care for self and or others, and used cross sectional data which ignore temporal changes such as adaptive strategies that are likely to influence individual responses to vision loss. Thus, additional larger longitudinal studies are clearly warranted to better delineate the role of sensory disability in handicap.
The self reported reduction in the capacity of people with visual impairment to act as carers for others when acuity in the better seeing eye is worse than 0.8 logMAR is also deeply worrying. Most studies9,11,13,22–25 have examined the impact of disability on the individual themselves; however, the consequences of sensory impairment in elderly carers has not been widely investigated. Our findings suggest that this is an area which warrants further investigation, particularly since the prevalence of moderate visual impairment in older people is high.2627
The findings of this study are particularly relevant for the generation of health economic models when assessing the value of management strategies in exudative AMD, and suggest that treatments which maintain vision at a level equal to or better than 0.9 logMAR are likely to result in savings in resource use by the avoidance of costs of care in visually impaired people themselves. Furthermore, treatments which result in maintenance of vision at a level of 0.7 logMAR or better may reap benefits through prevention of institutionalisation of the dependants of visually impaired people.
Acknowledgments
The authors gratefully acknowledge the support of the Guide Dogs for the Blind and the Medical Research Council, Strategic project grant G 9404235. The authors are also grateful to Ms M McClure, senior optometrist, Royal Hospitals Belfast and Ms Alyson Muldrew, Department of Ophthalmology, Queen’s University Belfast, for assistance with the database.
Abbreviations
AMD, age related macular degeneration
DVA, distance visual acuity
REFERENCES
- 1.Melser D, McWilliams B, Brayne C, et al. Profile of disability in elderly people: estimates from a longitudinal population study. Medical Research Council Cognitive Function and Ageing study (MRC CFAS) and Resource implications Study (RIS MRC CFAS). BMJ 1999;318:1108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson RJ, Wolinsky FD. The structure of health status among older adults: disease, disability, functional limitation and perceived health. J Health Soc Behav 1993;34:105–21. [PubMed] [Google Scholar]
- 3.Guralnik JM. The impact of vision and hearing impairments in old age. J Am Geriatr Soc 1999;47:1029–31. [DOI] [PubMed] [Google Scholar]
- 4.Leibowitz HM, Krueger DE, Maunder LR. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration and visual acuity in a general population of 2631 adults. 1973–1975. Surv Ophthalmol 1980;24:335–610. [PubMed] [Google Scholar]
- 5.Elliott DB, Rrukkolo M, Strong JG, et al. Demographic characteristics of vision-disabled elderly. Invest Ophthalmol 1997;38:2566–75. [PubMed] [Google Scholar]
- 6.Owen CG, Fletcher AE, Donoghue M, et al. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom? Br J Ophthalmol 2003;87:312–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wormald RP, Wright LA, Courtney P. Visual problems in the elderly population and implications for services. BMJ 1992;304:1266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans J, Wormald R. Is the incidence of registrable age related macular degeneration increasing? Br J Ophthalmol 1996;80:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams RA, Brody BL, Thomas RG, et al. The psychosocial impact of macular degeneration. Arch Ophthalmol 1998;116:514–20. [DOI] [PubMed] [Google Scholar]
- 10.Klein BEK, Moss SE, Klein R, et al. Associations of visual function with physical outcomes and limitations 5 years later in an older population. The Beaver Dam Eye Study. Ophthalmology 2003;110:644–50. [DOI] [PubMed] [Google Scholar]
- 11.Kuyk T, Elliott JL. Visual factors and mobility in persons with age-related macular degeneration. J Rehab Res Dev 1999;36:303–12. [PubMed] [Google Scholar]
- 12.Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF36). I: Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 13.Hart PM, Chakravarthy U, Stevenson MR, et al. A vision specific functional index for use in patients with age related macular degeneration. Br J Ophthalmol 1999;83:1115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery AM, Hart PM, Stevenson MR, et al. Validation and assigning of subscales to the Daily Living Tasks dependent on vision visual functioning questionnaire (submitted).
- 15.Jenkinson C, Layte R, Wright L, et al. The UK SF-36: an analysis and interpretation manual. University of Oxford, 1996.
- 16.McClure ME, Hart PM, Jackson AJ, et al. Macular degeneration: do conventional measurements of impaired visual function equate with visual disability? Br J Ophthalmol 2000;84:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsitz LA, Jonsson PV, Kelley MM. Causes and correlates of recurrent falls in ambulatory frail elderly. J Gerontol 1991;46:M114–22. [DOI] [PubMed] [Google Scholar]
- 18.Ivers RQ, Cumming RG, Mitchell P, et al. Visual impairment and falls in older adults: the Blue Mountains Eye Study. Am J Geriatr Soc 1998;46:58–64. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz A. The prevalence and consequences of visual impairment among nursing home residents. Monograph. New York: Lighthouse Inc, 1988.
- 20.Whitmore WG. Eye disease in a geriatric nursing home population. Ophthalmology 1989;96:393. [DOI] [PubMed] [Google Scholar]
- 21.Cumming RG, Salkeld G, Thomas M, et al. Prospective study of the impact of fear of falling on activities of daily living, SF36 scores, and nursing home admission. J Gerontol A Biol Sci Med Sci 2000;55:M299–305. [DOI] [PubMed] [Google Scholar]
- 22.Mangione CM, Gutierrez PR, Lowe G, et al. Influence of age-related maculopathy on visual functioning and health related quality of life. Am J Ophthalmol 1999;128:45–53. [DOI] [PubMed] [Google Scholar]
- 23.Scott IU, Smidde WE, Schiffman J. Quality of life of low vision patients and the impact of low vision services. Am J Ophthalmol 1999;128:54–62. [DOI] [PubMed] [Google Scholar]
- 24.Wang JJ, Mitchell P, Smith W, et al. Impact of visual impairment on use of community support services by elderly persons: The Blue Mountains Eye Study. Invest Ophthalmol Vis Sci 1999;40:12–19. [PubMed] [Google Scholar]
- 25.Evans JR, Fletcher AE, Wormald RPL, et al. Prevalence of visual impairment in people aged 75 years and older in Britain: results from the MRC trial of assessment and management of older people in the community. Br J Ophthalmol 2002;86:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Pols JC, McGraw PV, Thompson JR, et al. Visual acuity measurements in a national sample of British elderly people. Br J Ophthalmol 2000;84:165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]